This term includes all sympathetic nervous system tumours of neuroblastic origin. It comprises a wide spectrum of continuous morphological features, ranging from undifferentiated neuroblastoma to ganglioneuroma, depending on the proportion of neuroblastematous (NB) and ganglioneuromatous (GNR) components.
Clinical features
- It is the third most common extra cranial solid tumour of the paediatric age group.
- In 85% of the cases, it occurs in children under four years of age, and 50% under two years of age
- 65% of the cases present as an abdominal mass, with calcifications; although the adrenal is the most common site (50-80%), it can arise from any site containing sympathetic neural tissue
- Symptoms depend on its location: huge abdominal masses give rise to abdominal distension and respiratory symptoms; retroperitoneal masses can extend along the nerves and vertebral openings into the spine cord, resulting in pain and paralysis (Dumbbell)
- Nonspecific symptoms: fever, irritability, anorexia and malaise
- Para neoplastic syndromes may be related to other hormonal substances produced by the neuroblastoma or to an immune response (e.g. antibodies against the tumour) as a cause of myoclonus: opisthotonus, Horner syndrome or Ondine’s curse
- Metastases to regional lymph nodes, liver or bones at the time of diagnosis are common
- Metastases to the lungs are rare
- Catecholamine secretion: 24h urine specimens should be tested for homovanillic acid (HVA) and vanillylmandelic acid (VMA), before or soon after excision, for future follow-up and as an indicator of differentiation and survival.
- Familial incidence is reported
- Associations with Beckwith-Wiedemann Syndrome, Hirschsprung’s disease or neurofibromatosis, or as a complication of foetal hydantoin syndrome.
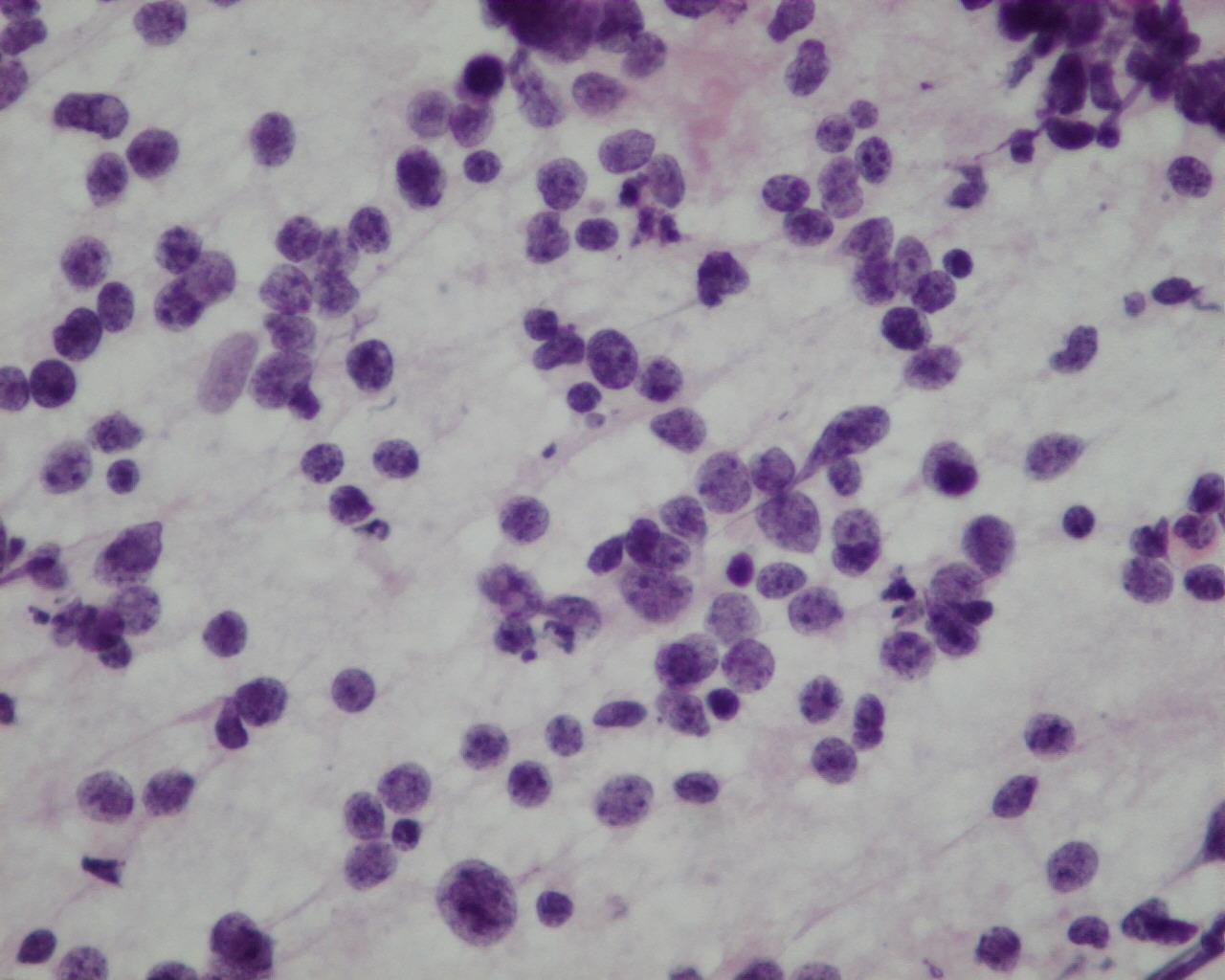
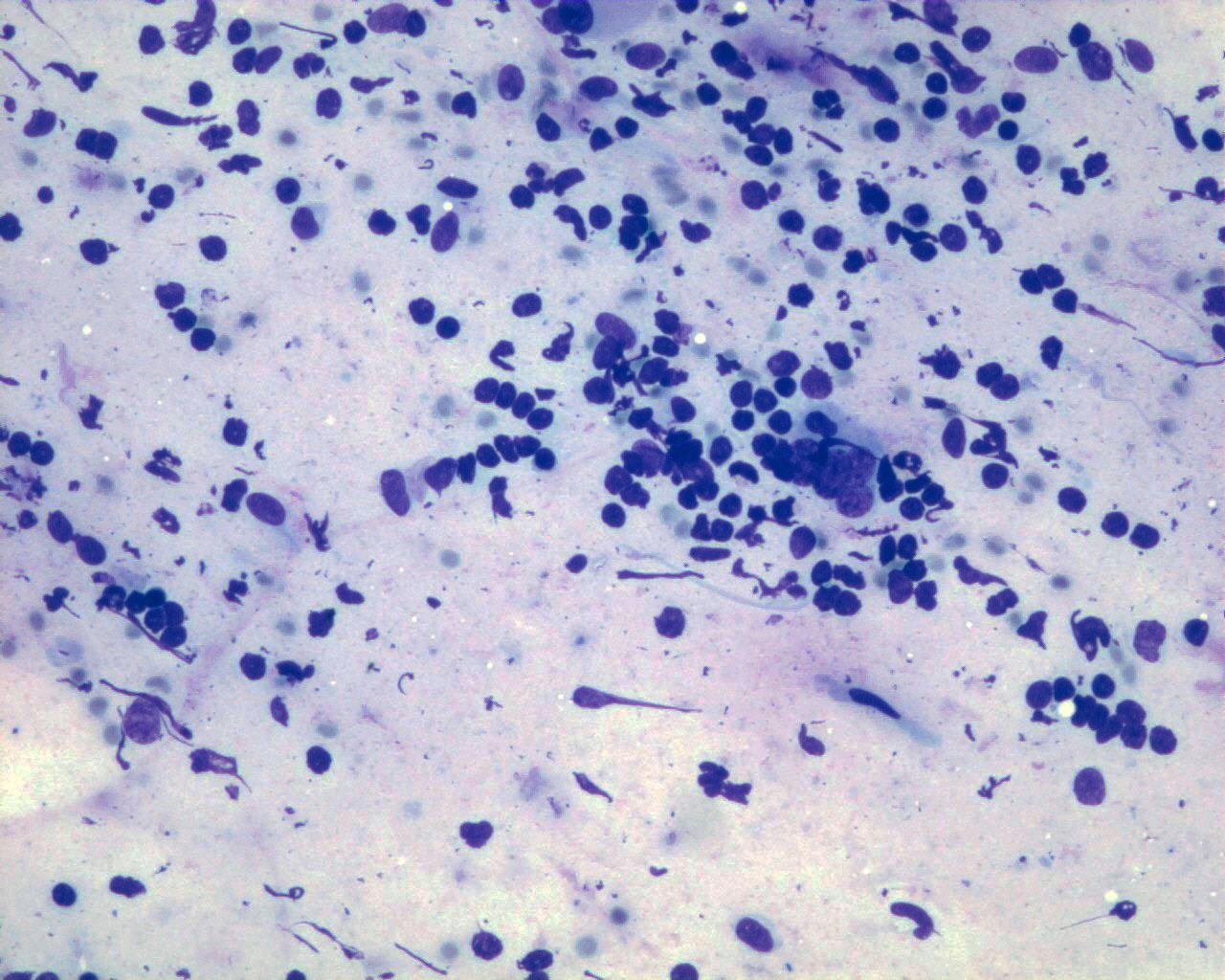
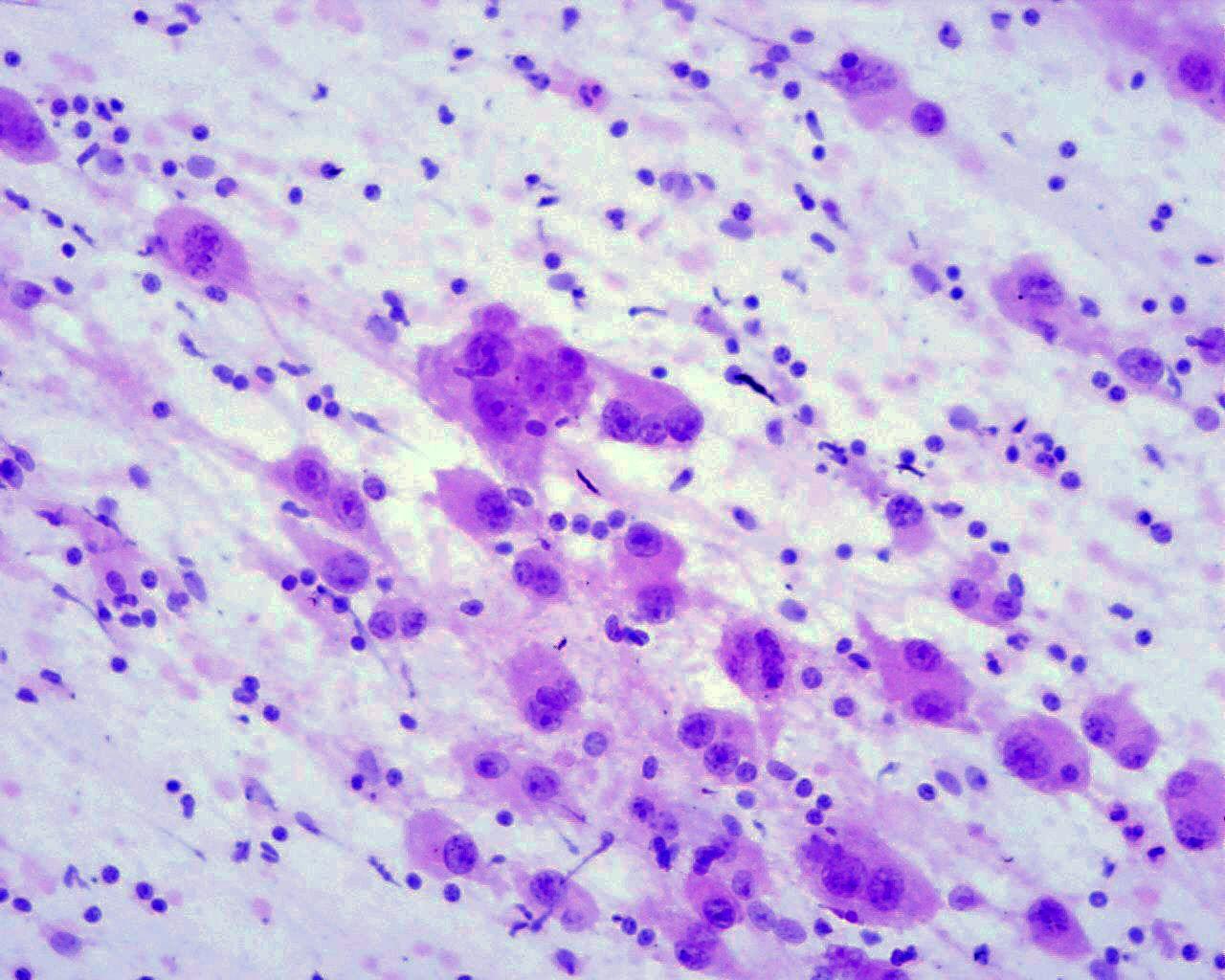
Fig 43a – Neuroblastoma NOS – Neuroblastoma poorly differentiated- Smears consisting mainly of neuroblastematous component. Small round cells with very high nuclear-cytoplasmic ratio, granular “salt and pepper” chromatin and inconspicuous nucleoli in fine fibrillary background. Absence of neuroblastic differentiation (H&E)
- Distinguishing between the different types of neuroblastoma and ganglioneuroblastoma (INPC-classification) is impossible by fine-needle cytology, since this depends on the architecture and on exhaustive sampling of the tumour
- Its appearance depends on whether the NB or the GNR component predominates.
- neuroblastematous (NB) component :
- Hypercellular smears
- Undifferentiated loose, round, small cells
- Undifferentiated neuroblasts have round to oval nucleus with granular salt-and-pepper chromatin and inconspicuous nucleoli
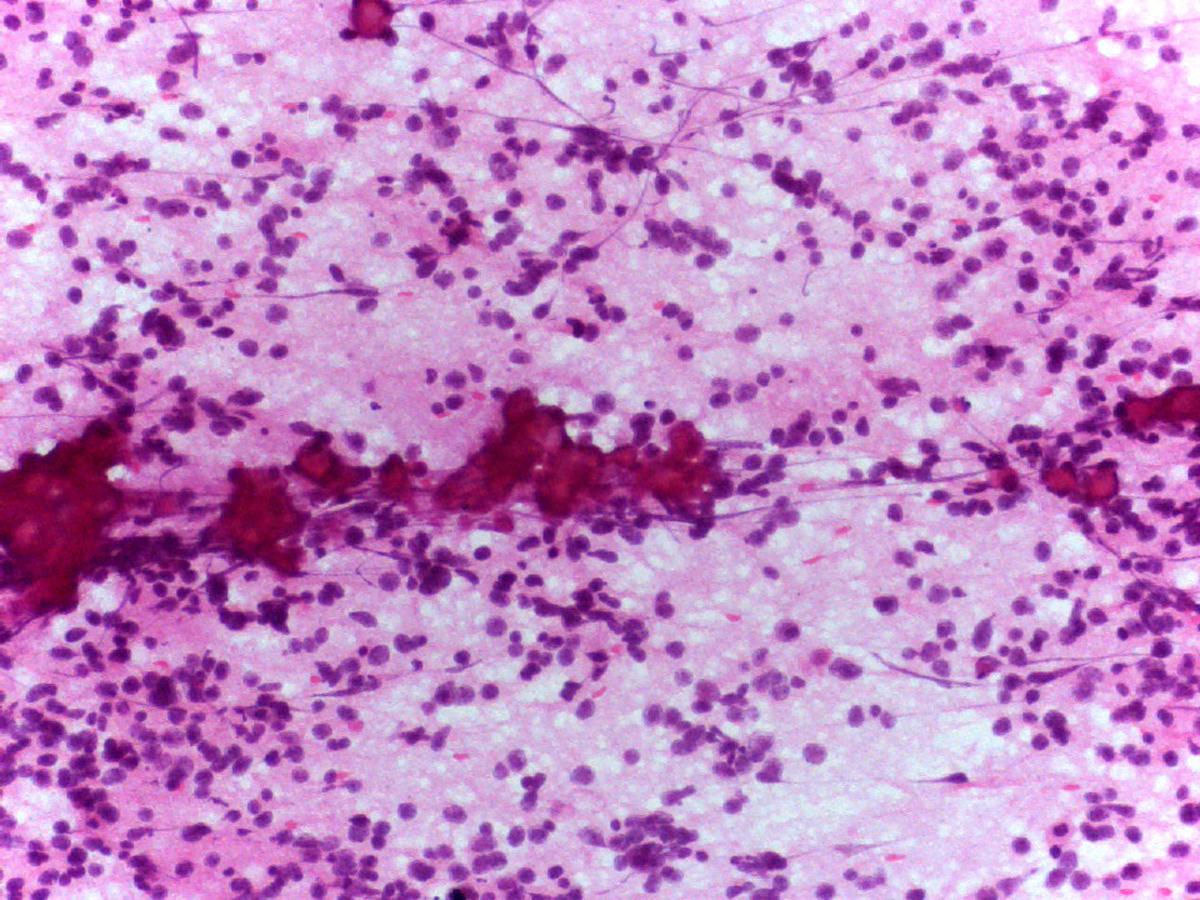
- Background of fibrillary material
- Mitoses are frequent
- Necrosis may be abundant
- ganglioneuromatous (GNR) component:
- Feeling of hardness while puncturing
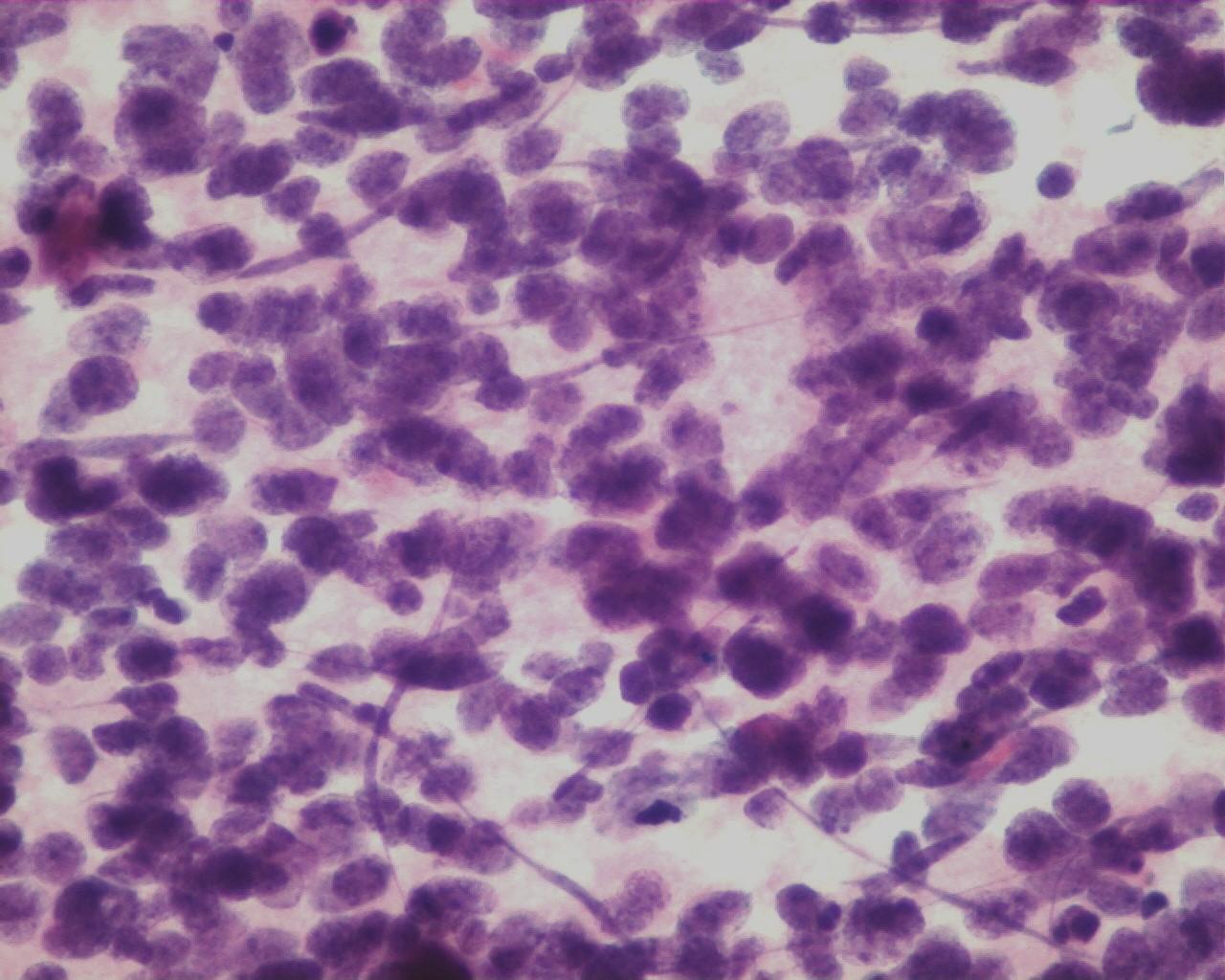
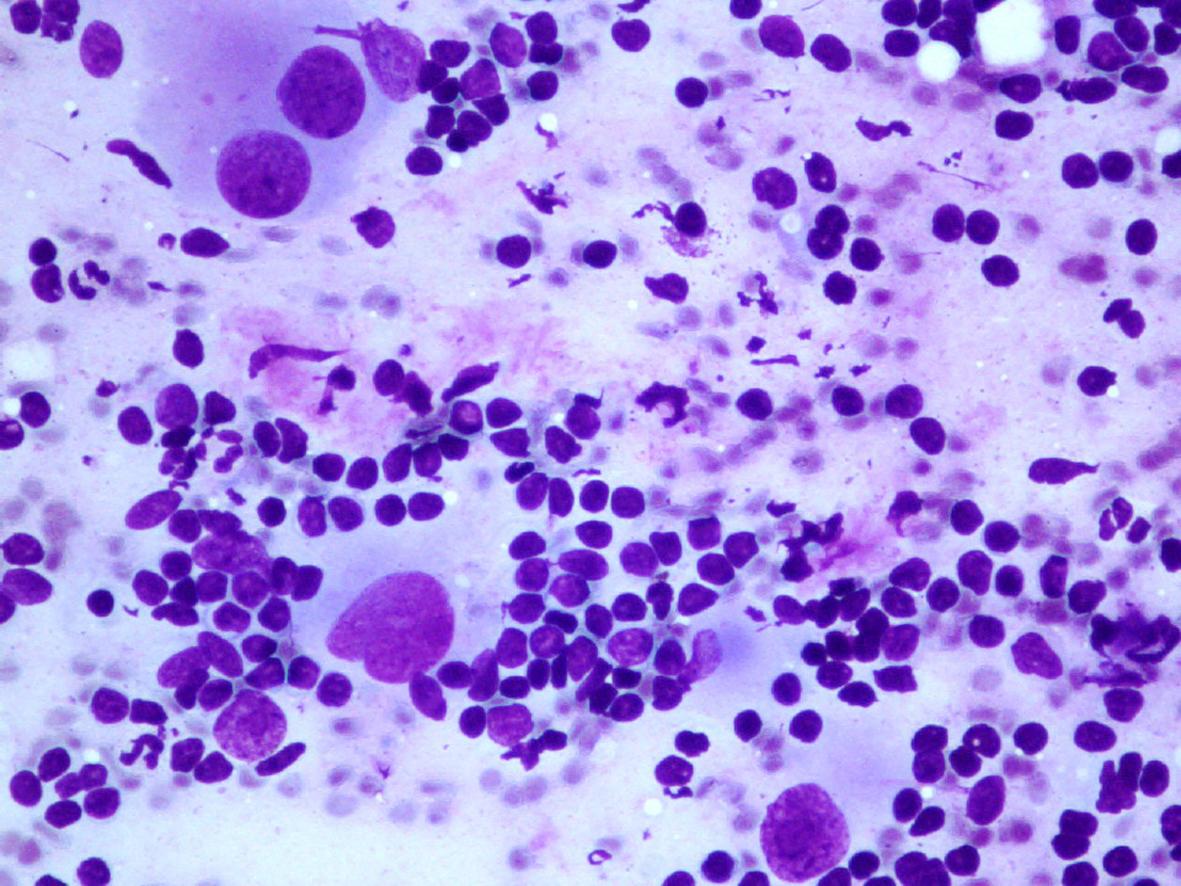
- Hypocellular smears
- Mature or maturing ganglion cells
- Rare spindle nucleus, corresponding to Schwann cells
- Collagen matrix
- neuroblastematous (NB) component :
- Although these two components are essential for classifying neuroblastic tumours, the cytological appearance more frequently has an intermediate pattern:
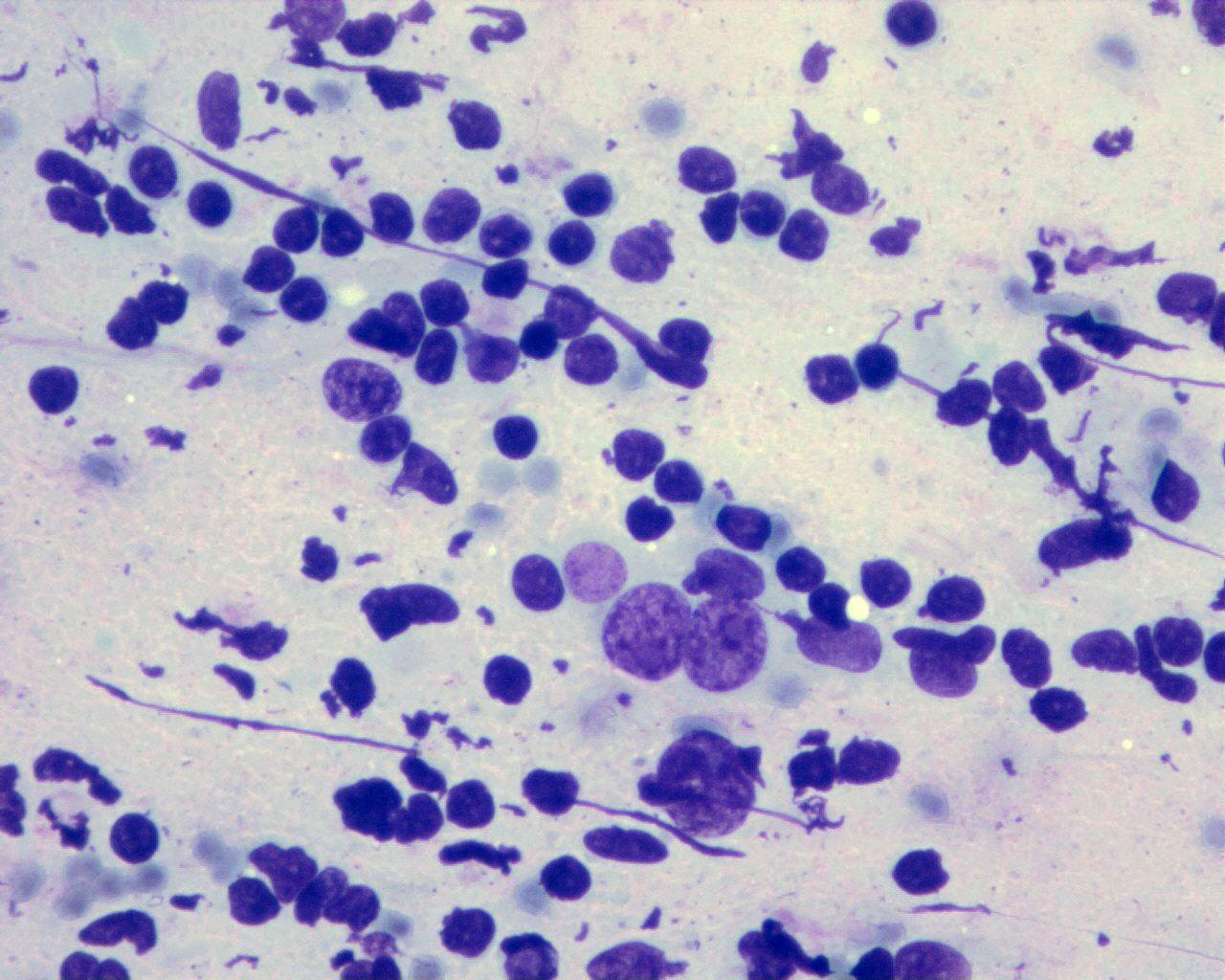
- Neuroblasts at varying degrees of maturation:
- Undifferentiated neuroblasts
- Small uncommitted round cells
- Maturing neuroblasts
- Neuroblasts with eccentric enlarged nucleus
- Small eosinophilic nucleoli
- Enlarged well-defined cytoplasm with Nissl substance
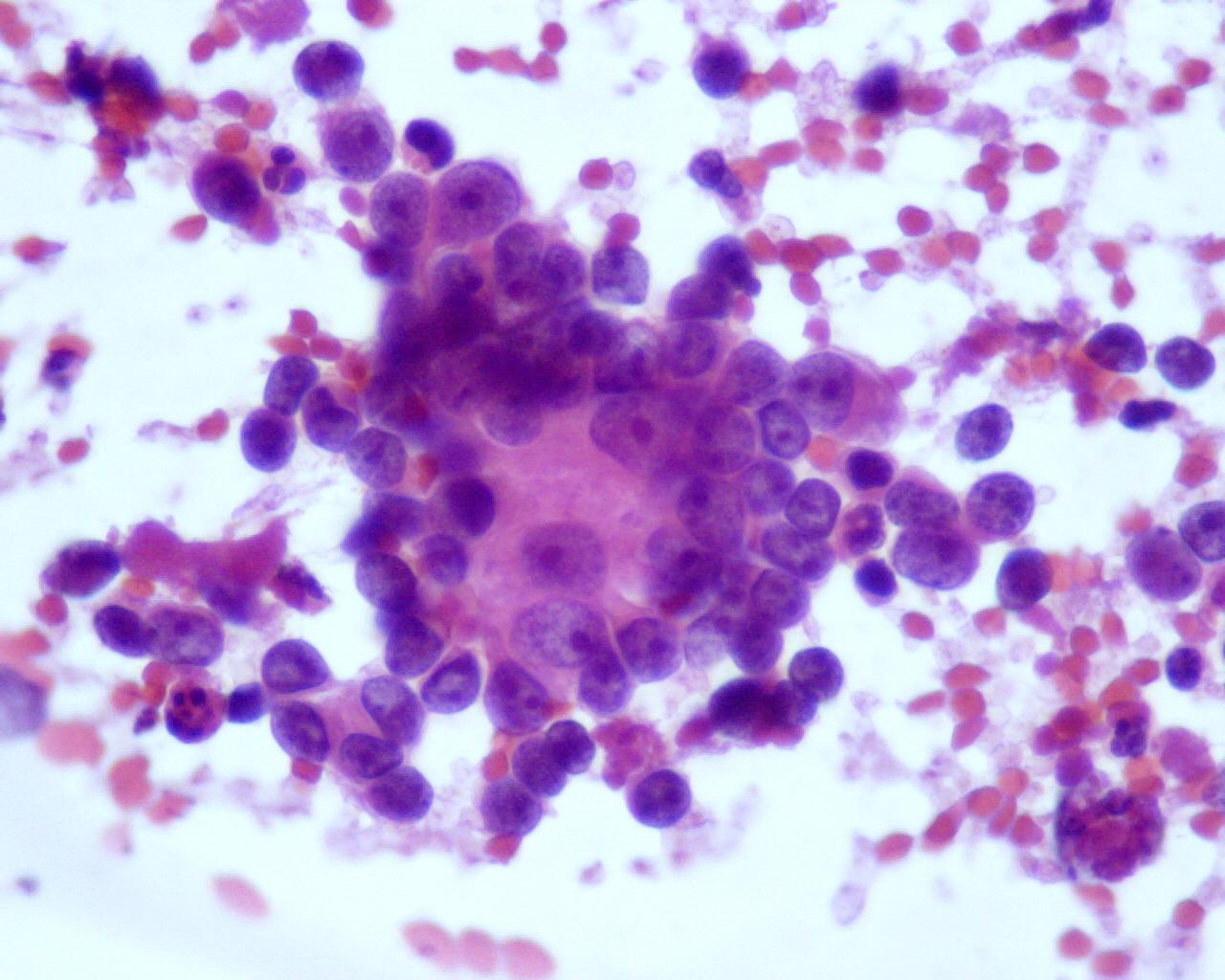
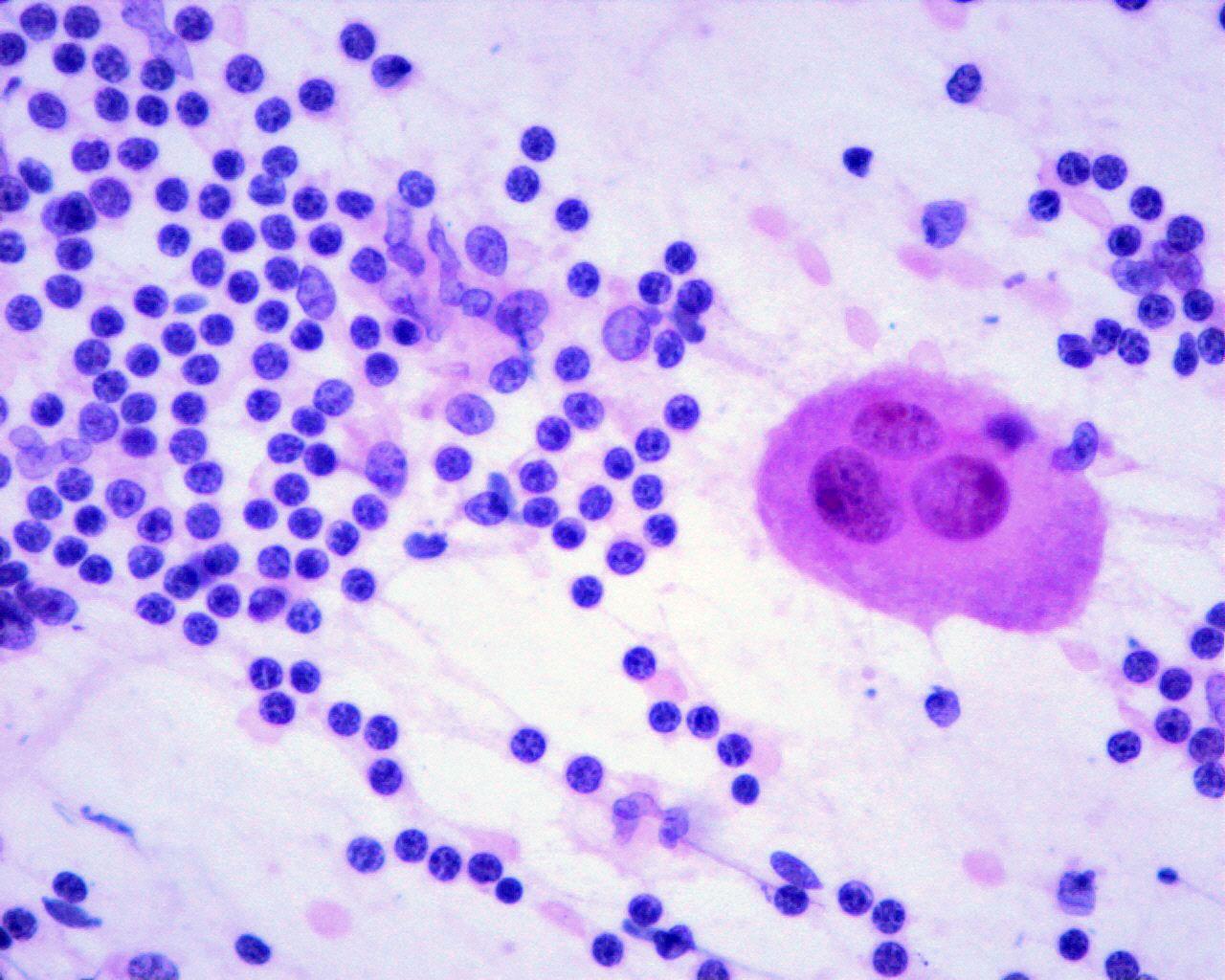
- Immature ganglion cells
- Frequent binucleation
- Eccentric huge vacuolated nuclei with prominent eosinophilic nucleoli
- Enlarged well-defined cytoplasm with Nissl substance
- Undifferentiated neuroblasts
- Variable amount of fibrillary neuropil (specific characteristic)
- Homer-Wright rosettes (not a specific characteristic, but they occur at a higher frequency than in other small cell tumours)
- Sparse Schwann cells
- Variable numbers of mitoses and necrotic cells
- Calcifications can be seen
- Neuroblasts at varying degrees of maturation:
[Table 1]
International Neuroblastoma Pathology Classification
(International Neuroblastoma Pathology Committee (INPC) classification)
*Original Shimada system (SR = stroma-rich; SP = stroma-poor; SD = stroma-dominant)
**INPC grade (FH = favourable histology; UH = unfavourable histology)
- *** GNBn:
- Composite tumour with a favourable stroma-rich/stroma-dominant component and a nodular component of either a biologically favourable clone or an unfavourable clone, or both.
- In a recent study on 70 patients with GNBn by Umehara et al (6), the clinical behaviour (FH/UH) was found to be connected with the grade of the most unfavourable component present in the tumour.
- Large cell type phenotype – (large nuclei, clear chromatin, sharp nuclear membranes, and prominent nucleoli (7% of the cases); confers bad prognosis.
Immunocytochemistry
- CD56 N-CAM: positive
- NB84: positive
- NSE: positive
- Synaptophysin: positive
- Chromogranin A: positive
- Leu7: positive
- GD2 (only in frozen material): positive
- CD117: positive (50%)*
- Vimentin: negative
- CD99: negative
- Actin: negative
- Desmin: negative
- Cytokeratin: negative
*some authors associate to better prognosis
Genetic studies:
- N-myc expression and amplification
- Ploidy
- Deletion of chromosome 1
- 17q gains
- Double minute chromosomes
- Homogeneous staining regions
- TrKA, TrKB and TrkC expression (receptor proteins)
Differential diagnosis
- Lymphoma
- Lymphoglandular bodies
- Lack of fibrillary neuropil in the background
- Lack of rosette formation
- Neuroepithelial markers (NSE, CD56 N-CAM, synaptophysin and others): negative
- CD45: positive
- Rhabdoid tumour
- More monotonous cellular population
- Vesicular chromatin
- Homogeneously prominent nucleoli
- Cytokeratin: positive (dot)
- Vimentin: positive (dot)
- INI : negative
- Alveolar rhabdomyosarcoma
- Myogenin: positive
- N-myc amplification (may be detected)
- Desmoplastic small cell tumour
- Desmin: positive (dot)
- Cytokeratin: positive
- Vimentin: positive
- t(11;22)(p13;q12)
- PNET
- Most common in young adults.
- Absence of fibrillary neuropil
- Dual population of light and dark cells
- Cytoplasm glycogen
- CD99: positive (membranous pattern).
- t (11:22) (q24; q12).
Main points
- Poor prognostic indicators: age over 1.5 years, 1p36,33 deletion, 14p deletion, N-myc amplification, diploid, low expression of TrKA (maturation factor), undifferentiated morphology, high MKI and CD44 positivity (correlates with N-myc amplification: the greater the number of copies of N-myc is, the worse the prognosis will be)
- Favourable prognostic indicators: age under one year, hyperdiploid/near-triploid, high levels of TrKA gene and no N-myc amplification
- Intermediate prognostic indicators: older patients, near diploid/tetraploid, low levels of TrKA gene, no N-myc amplification and no 1p deletion
- TrKB expression: generally expressed in advanced tumours
- Stage IVs (any localized tumour, with liver or bone marrow metastasis, but with no bone involvement); these cases are generally seen in infants and usually have a good prognosis
- Neuroblastoma in situ: incidental finding at autopsy in infants less than three months of age
- Extra-adrenal tumours are better differentiated and have better prognosis
- Low urinary VMA/HVA ratio is associated with poor outcome (sign of lack of differentiation)