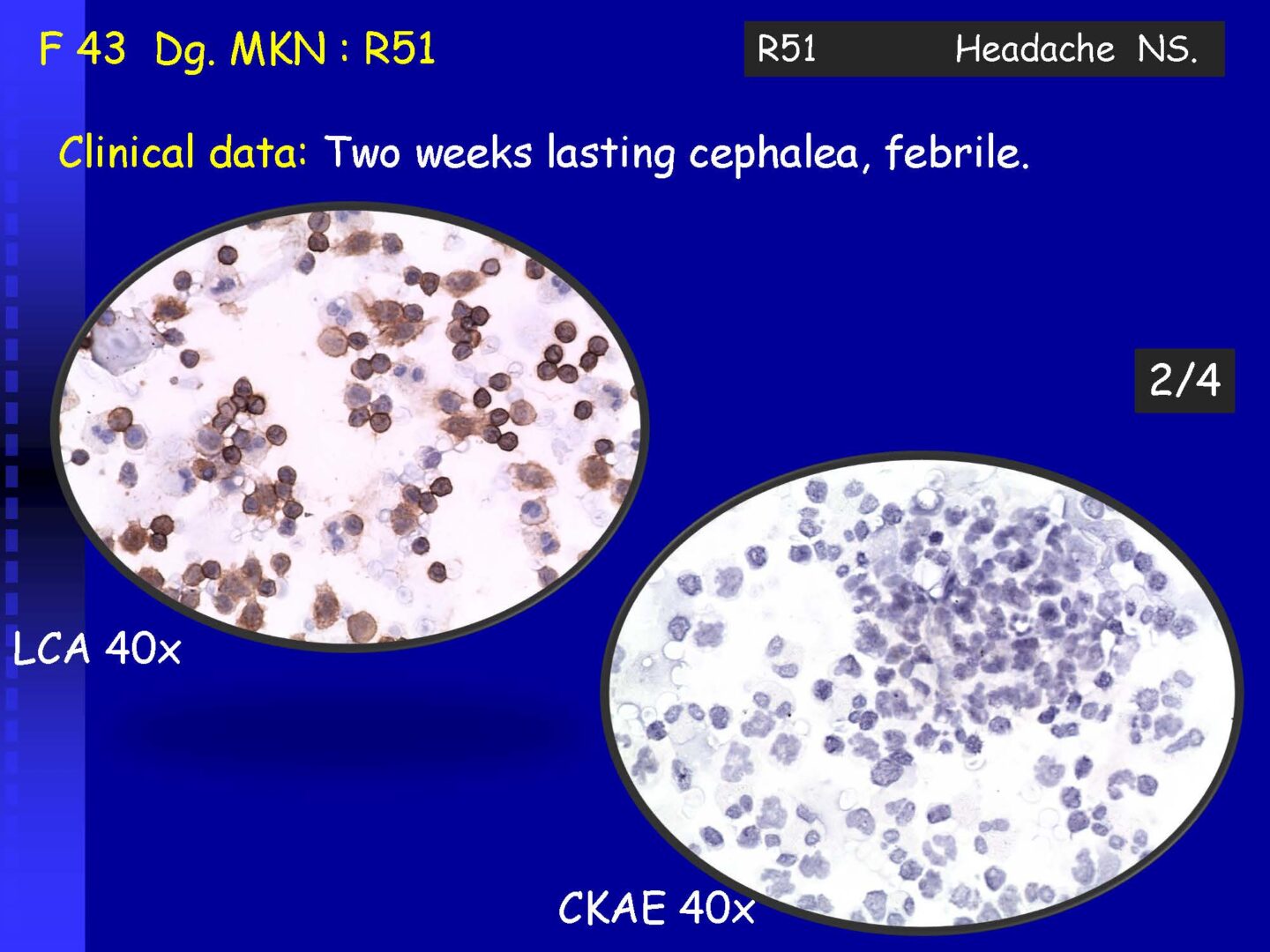
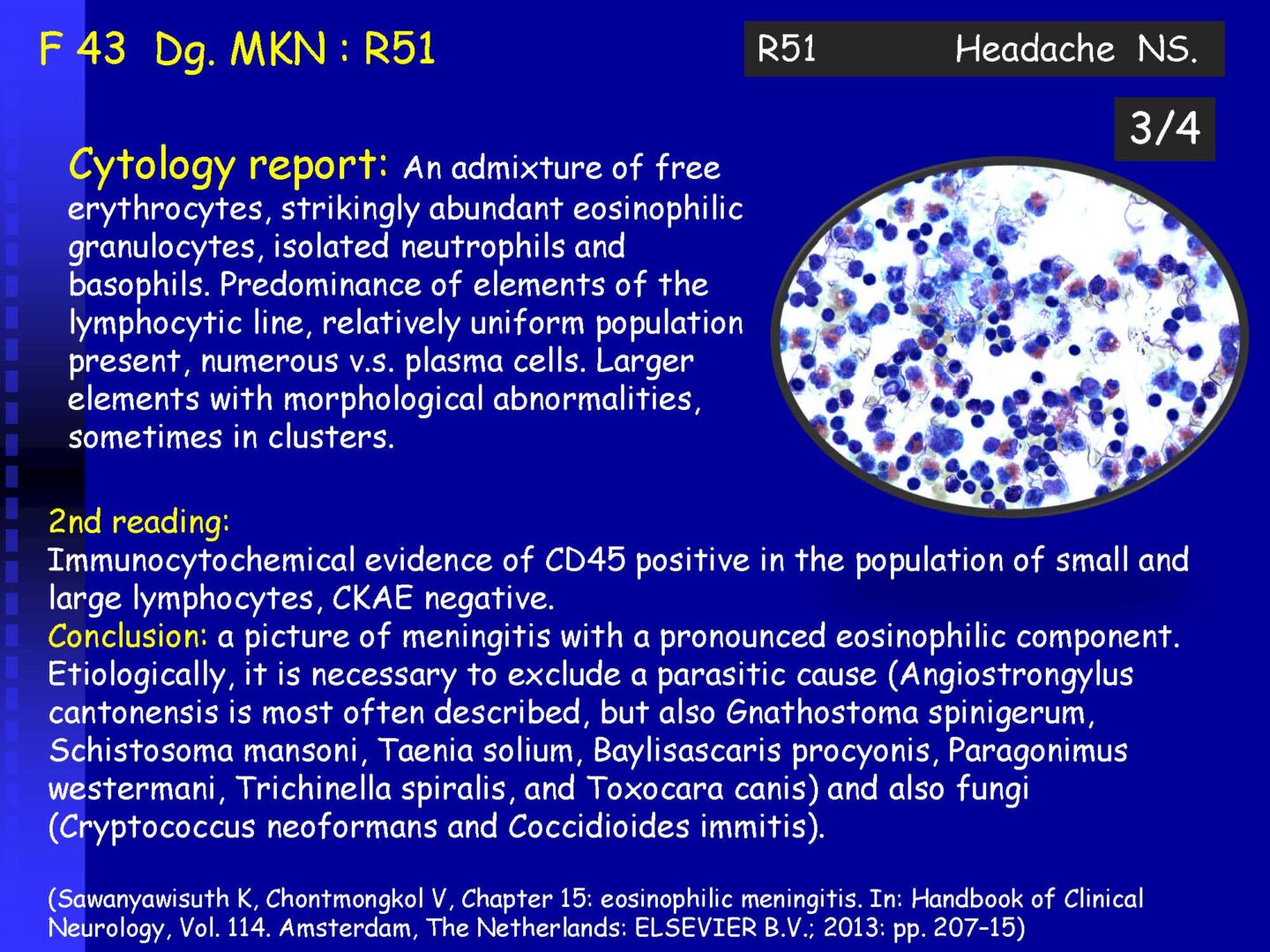
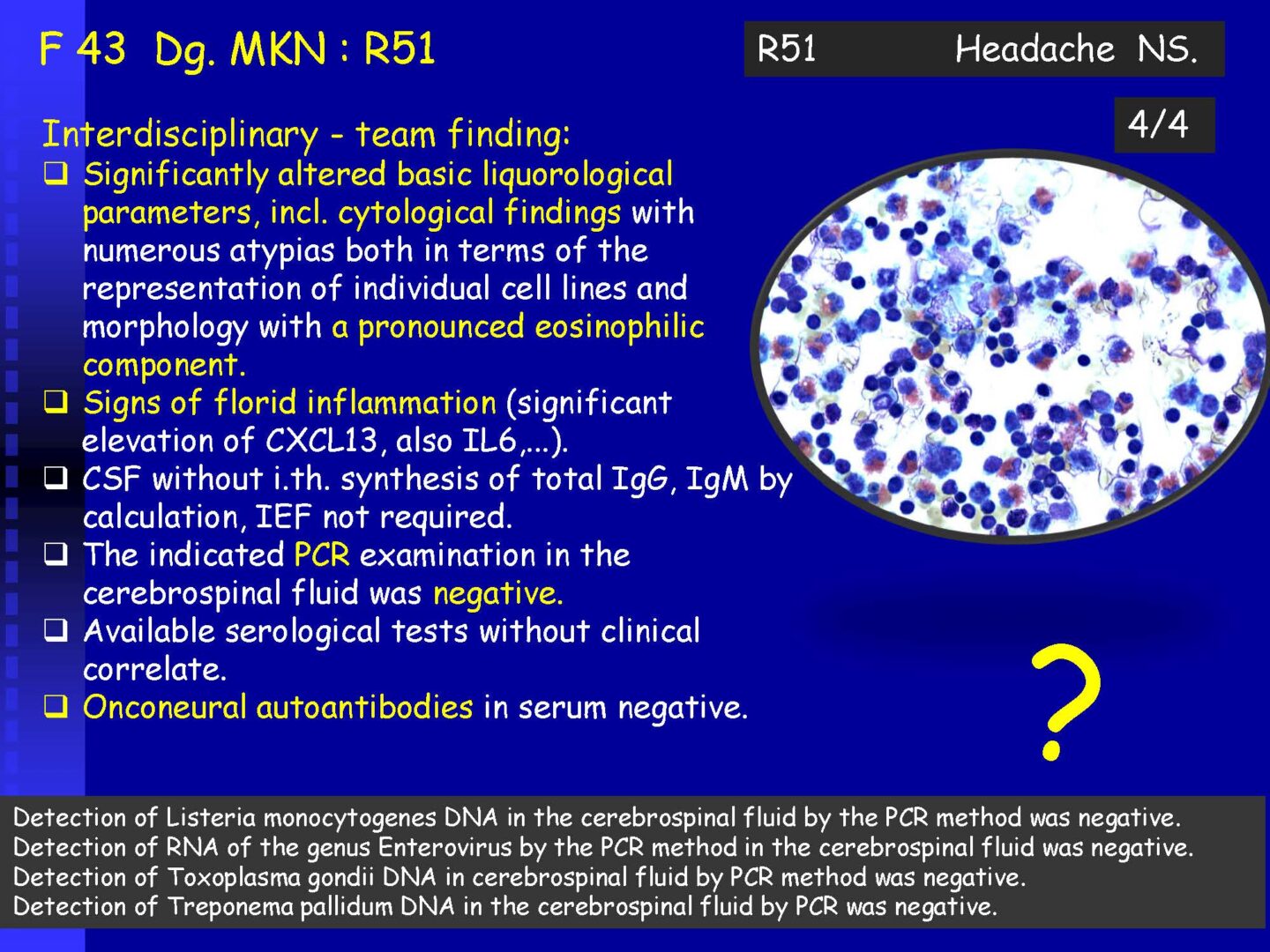
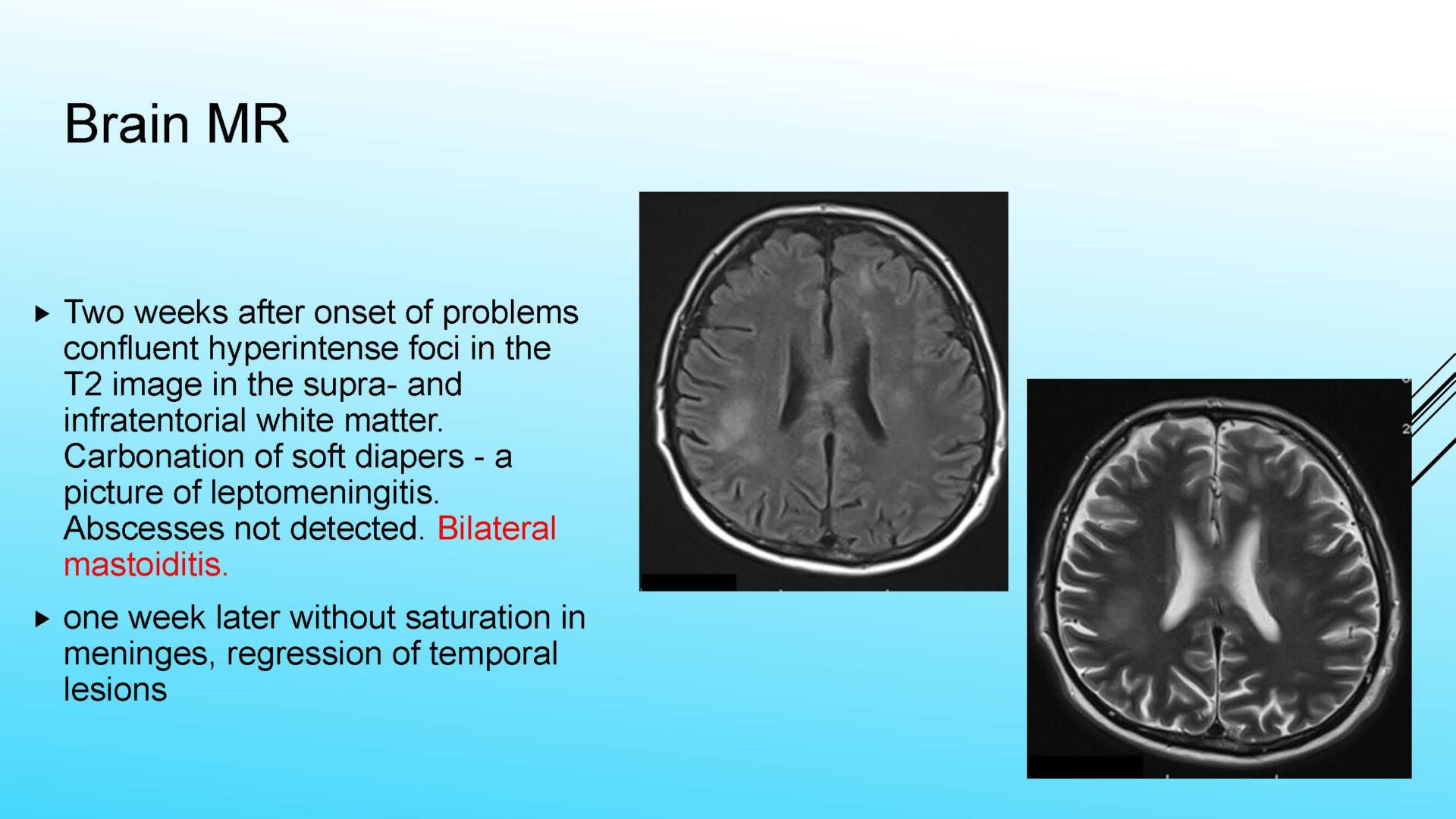
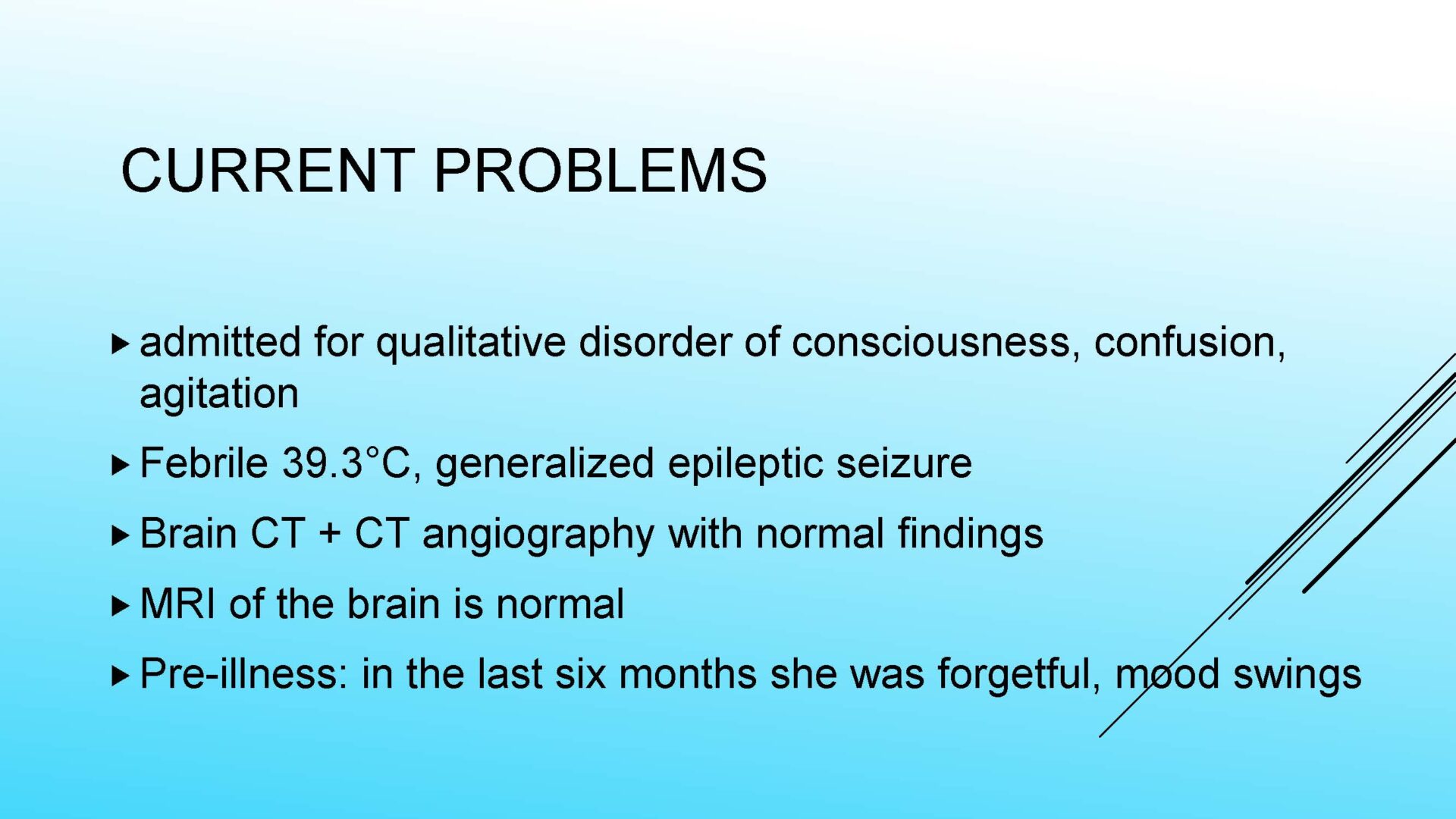
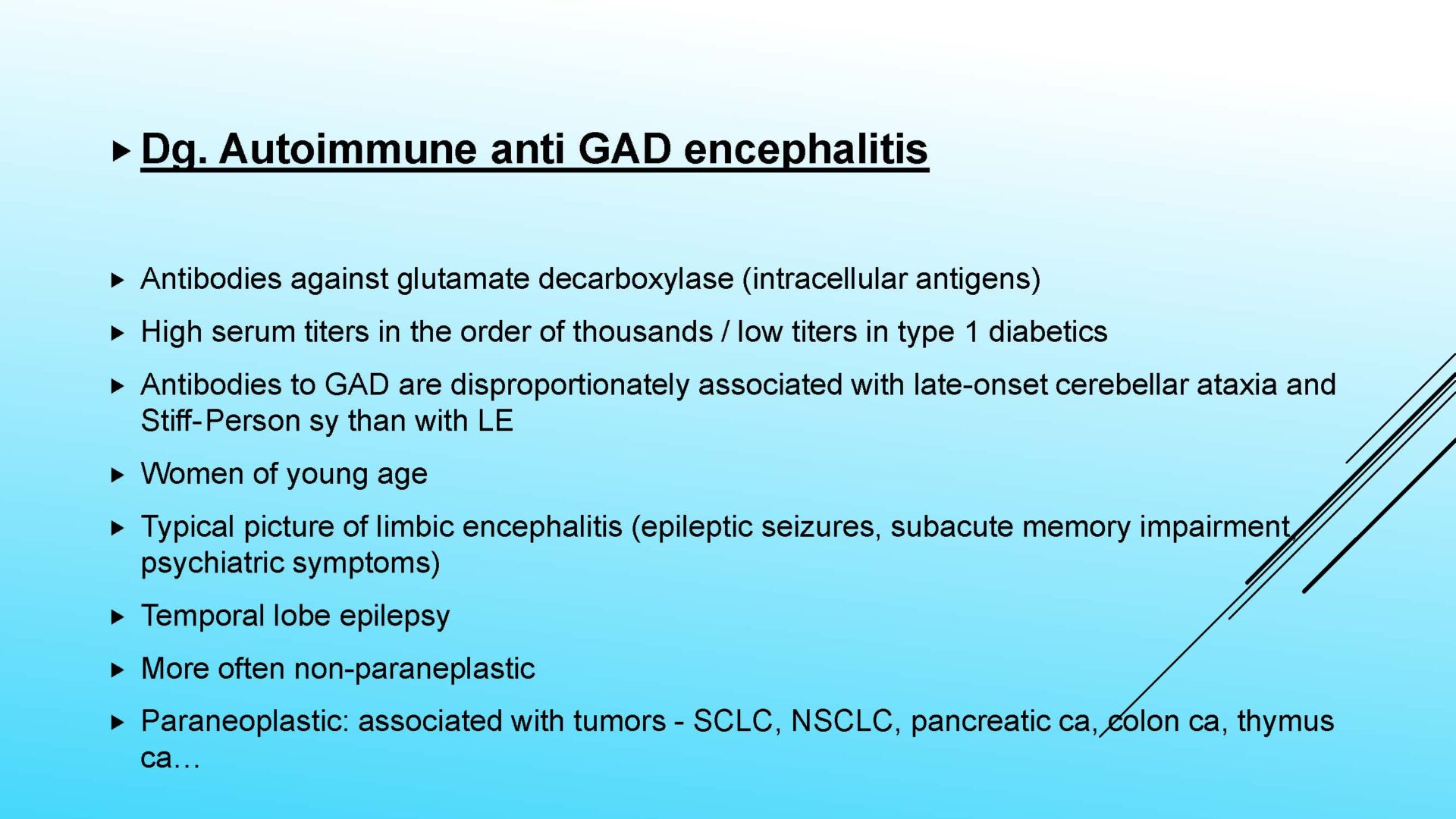
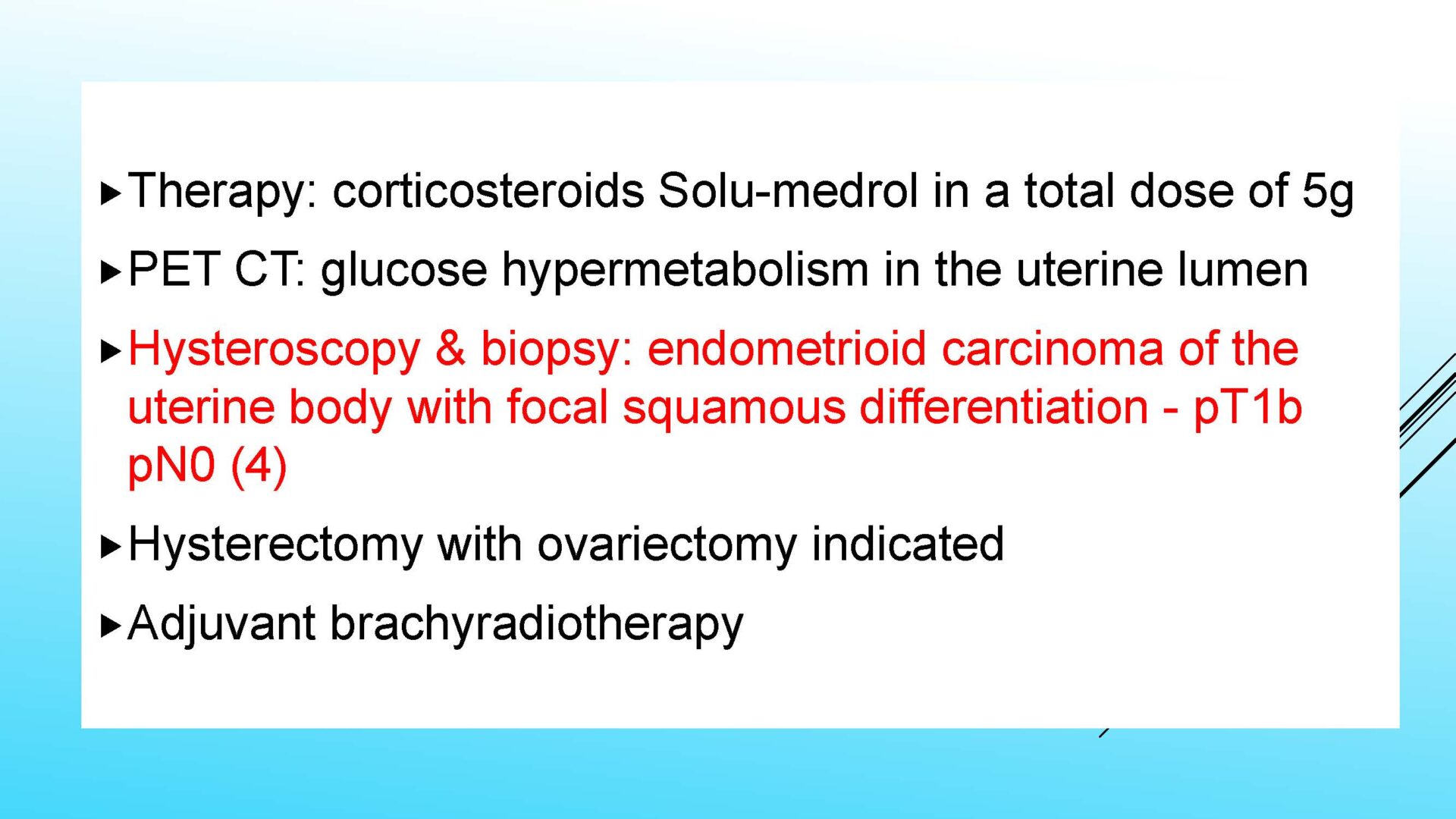
On completion of this section the cytotechnologist / cytopathologist should know:
Anatomical, physiological and clinical introduction
the anatomy and physiology of the CSF and its production
the indications and contraindications of the CSF examination
the technique(s) of the spinal tap
the rules for the pre-analytical phase of the CSF investigation
Diagnostic tests in CSF
cytopathological:
evaluation of cellularity and erythrocytes contamination
slide preparation (including conservation of reserve slides for subsequent special techniques)
basic staining
additional techniques, especially immunocytochemistry, and flow cytometry
the quantitative and qualitative criteria for a satisfactory CSF sample
the terminology for reporting results
nonmorphological methods –the complex approach to the CSF investigation:
Biochemical
Immunological
Microbiological
Special CSF diagnostics
the benign diseases –clinical and cytopathologic features
inflammatory diseases of the Central Nervous System (CNS)
infectious inflammations of the CNS
purulent bacterial neuroinfections
serous/ aseptic bacterial and viral neuroinfections
mycotic, parasitic neuroinfections
autoimmune inflammations of the CNS
multiple sclerosis and other demyelinating diseases
AIDP (acute demyelinating polyneuropathies) / CIDP (chronic demyelinating polyneuropathies)
Other autoimmune diseases
brain and spinal cord tissue lesions
Traumatic Brain Injury (TBI)
CSF changes after neurosurgery
spinal cord and radicular compression
vascular diseases of the CNS
intracranial bleeding, SAH
ischaemic stroke
degenerative diseases of CNS
tumorous impairment of the CNS
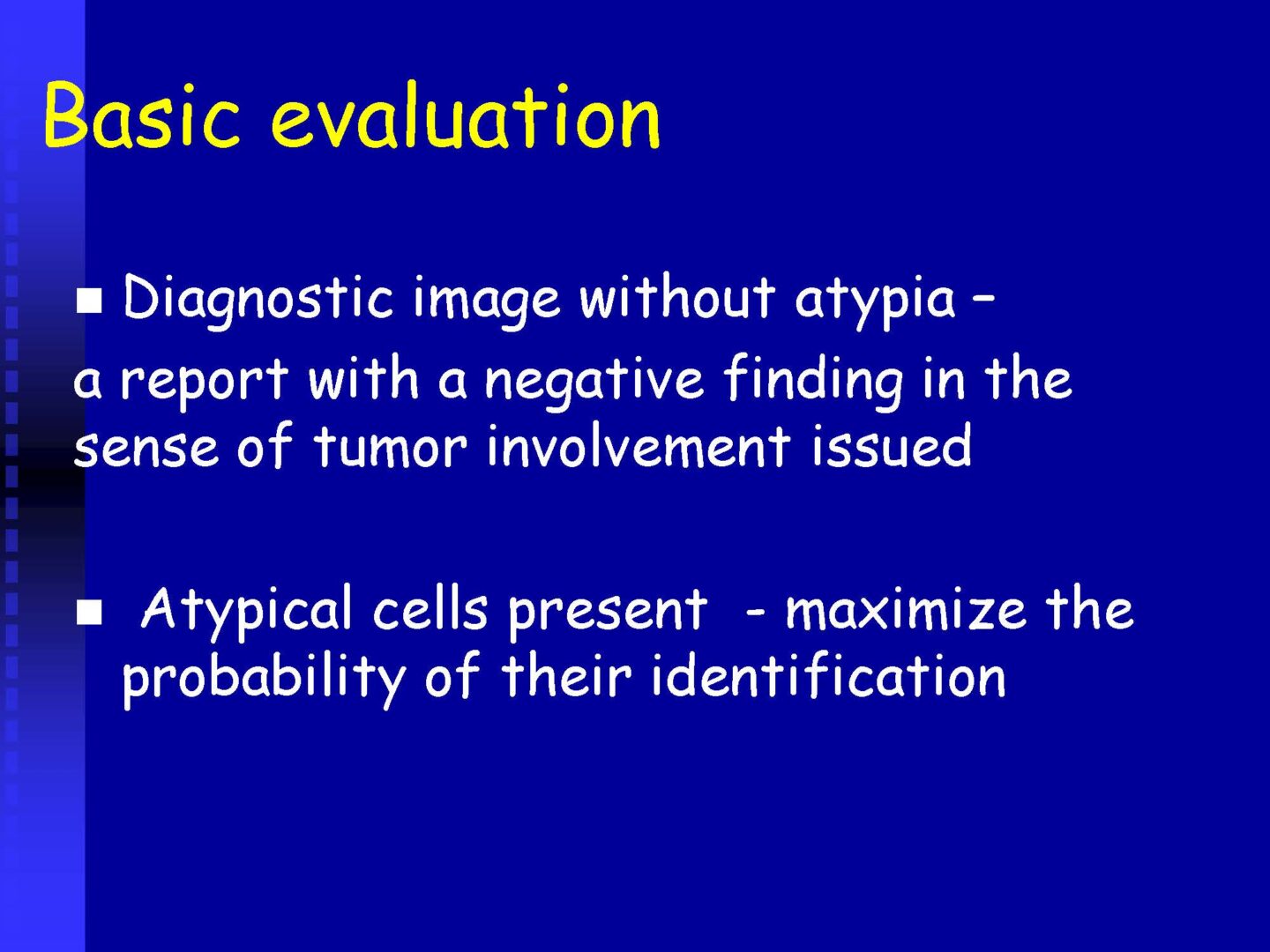
the criteria to evaluate a sample as atypical
the criteria to evaluate a sample as suspicious for malignancy
metastatic
haematological
primary brain neoplasms
the criteria to evaluate a sample as malignant
metastatic
haematological
primary brain neoplasms
Proposed standardized categories for the CSF sample evaluation:
Non-diagnostic – Unsatisfactory ND/UNS
Benign – Negative for malignancy NFM
Atypia of undetermined significance AUS
Suspicious for malignancy SFN
Malignant
Prof. Jaroslava Dušková, MD, PhD, FIAC is a pathologist, based at the Department of Pathology, First Faculty of Medicine, Charles University and General University Hospital in Prague. Prof. Dušková is also the Head of the pathology and cytology department of Laboratory for CSF, neuroimmunology, pathology and special diagnostics, Topelex s.r.o.
Dr. Ondřej Sobek, MD, PhD, MIAC is board examined neurology specialist licensed for CSF cytology. He is the Head of Laboratory for CSF, neuroimmunology, pathology and special diagnostics, Topelex s.r.o.
The laboratory for CSF, neuroimmunology, pathology and special diagnostics, Topelex s.r.o., represents a unique multidiscipline centre in Prague, Czech Republic, focused on laboratory diagnostics, mainly of the cerebrospinal fluid in the areas of clinical biochemistry, immunology, microbiology and cytopathology.
The workplace follows and further develops a concept of Laboratory for CSF and Neuroimmunology pertaining to the Department of Neurology, First Faculty of Medicine, Charles University in Prague. In 2008, the laboratory was established as an independent legal subject, Topelex s.r.o. ( http://www.likvor.cz/en/ )
The laboratory Topelex has a significantly multiregional character and serves as an expert centre for CSF testing in the national system of external quality assessment in the Czech Republic (sekk.cz). The laboratory tests aprox. 7.000 samples of CSF annualy and is certified by ISO 15189 at Czech Accreditation Institute.
Cerebrospinal fluid (CSF) is a clear, colorless fluid in the tissues surrounding the brain and spinal cord of vertebrates.
A historical note
The presence of fluid in the brain was already known to Hippocrates and Galen, but they considered it a pathological phenomenon. The first truly scientific treatise on CSF was written between 1741-1744 by the Swedish mining engineer Emanuel Swedenborg (1688-1772) (Fig. 1), who, inspired by the search for underground water sources and his theological orientation, decided to find the seat of the soul. After a thorough study of the anatomy of the brain with the best anatomists in France, Germany and Italy, he wrote a scientific treatise on CSF. Due to the different profession, however, he did not find trust for his study with the approached publishers. His work was found, rightly appreciated and published in translation only 150 years later in 1887 [2]. The further development of the knowledge of anatomical details reminds us of the names that are immortalized in anatomical terminology: foramen Magendie, foramina Luschkae described by German anatomist Hubert von Luschka (1820-1875). French physiologist Francois Magendie (1783-1855) (Fig.2) is the author of the term cerebrospinal fluid. He also introduced measuerement of CSF pressure [3].
Lumbar punctures were already performed at the end of the 19th century [4].
In the twentieth century, several hundred-page monographs on CSF were produced [5,6].
The development of the Czech literature on CSF is sadly marked by the fate of the professor of the Czech part of Charles University, Leo Taussig (1884-1944) (Fig.3), a native of central Bohemia. His remarkably comprehensive 440-page monograph on cerebrospinal fluid written in Czech and published in Prague as early as 1926 [7] is practically unsearchable in world databases.
During the German occupation of Czechoslovakia Leo Taussig was deported to a fortress and camp Theresienstadt in 1942, perished in Auschwitz in 1944. His plan to translate the monograph into French and thus make it available to the scientific community remained unfulfilled and the work did not receive the recognition it deserved.
Taussig’s fate is tragically similar to that of Dr. Oskar Fischer – born in Slané (Bohemia) in 1876. He graduated in Prague in 1900, worked two years at the Institute of Pathology and Anatomy of the German Karl-Ferdinand University. Then at the Psychiatric clinic led by Arnold Pick. He described Neuritic plaques in detail in 1907 [8]. Oskar Fischer is also the author of the term pleocytosis of cerebrospinal fluid, which is still used today. Oskar Fischer died in Terezin 1942. His studies on dementia, now called Alzheimer’s, were appreciated much later [9, 10].
Research of the CSF space continues; during the last decade especially by studying signal cascades and relationships between cerebrospinal fluid and interstitial fluid [11-13].
References
Hajdu SI. A note from history: Discovery of the cerebrospinal fluid. Ann Clin Lab Sci 2003; 33(3): 334-336.
Wynter W. Four cases of tubercular meningitis in which paracentesis of the theca vertebralis was performed for the relief of fluid pressure. Lancet 1891; 137(3531): 981-982.
Tubbs RS et al. : Francois Magendie (1783-1855) and his contributions to the foundations of neuroscience and neurosurgery. J Neurosurg 2008, 108:1038-42
Quincke H. Die Lumbalpunction des Hydrocephalus. Berl klin Wochenschr 1891; 28(929-933; 965-968.
Merritt HH, Fremont-Smith F. The cerebrospinal fluid ed.). Philadelphia, London,: W. B. Saunders Company; 1937:
Herndon RM, Brumback RA. The cerebrospinal fluid ed.). Boston: Kluwer Academic Publishers; 1989:
Taussig L. Liquor cerebrospinalis: Lumbální punkce v diagnostice, prognostice a terapii. Praha: Mladá Generace Lékařů; 1926: 440.
Fischer O. Miliare Nekrosen mit drusigen Wucherungen der Neurofibrillen, eine regelmässige Veränderung der Hirnrinde bei seniler Demenz. Monatsschr Psychiat Neurol 1907; 22: 361–72.
Goedert M. Oskar Fischer and the study of dementia. Brain 2009; 132(Pt 4): 1102-1111.
Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 2011; 128(6): 309-316.
Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG. The role of brain barriers in fluid movement in the Cns: Is there a ‘glymphatic’ system? Acta Neuropathol 2018; 135(3): 387-407.
Naganawa S, Taoka T: The Glymphatic System: A Review of the Challenges in Visualizing its Structure and Function with MR Imaging. Magn Reson Med Sci 2022, 21:182-94.
Cerebrospinal fluid is a clear, colorless liquid in the amount of approximately 150 ml produced in the choroid plexuses (to a small extent also in the ependyma and endothelium) of the lateral cerebral ventricles with a volume of 25 ml; from them, it reaches the subarachnoid space through the foramen of Magendie and the foramen of Luschkae via the third and fourth cerebral ventricles – this is where the remaining 125 ml is located.
It is resorbed in the villi duroarachnoidales along the superior sagittal sinus.
The functions of the CSF
lightening the brain weight – 1400 g reduced by buoyancy to less than 50g
shock protection
reserve space for expansions
homeostasis – exchange of substances between brain cells
energy-metabolic – scavenger of free radicals
cleansing – removal of metabolic products
protection against pathogens [1]
The CSF volume renews four times in 24 hours.
Normal supine CSF pressure in an adult ranges between 10-15 mm Hg, 3-4 mm Hg in children [2].
Comment: Cerebrospinal fluid is a medium that is part of a specific and largely isolated body compartment of the CNS. The cerebrospinal fluid is in close contact with nervous tissue. Its relatively easy accessibility for laboratory examination makes it an important diagnostic matrix for pathological conditions of the CNS. Due to its intimate relationship with the CNS (i.e. closer to pathological processes in nervous tissue) and its relatively small volume (approx. 150 ml), CSF reflects biochemical, energy-metabolic, immunological and cellular processes in the CNS much more sensitively and specifically than possible blood tests. It is a completely common clinical situation when, with grossly pathological findings in the cerebrospinal fluid, we do not find any pathological alterations of the blood laboratory parameters. On the other hand, comparison of findings in CSF and serum can be diagnostically beneficial. Therefore, the simultaneous delivery of a blood and cerebrospinal fluid sample is an advantage especially when biochemical, microbiological or neuroimmunological laboratory testing is required (specified in the laboratory manual of the multidisciplinary laboratory for CSF analysis Topelex, s.r.o. – http://www.likvor.cz ). A comparison of physiological values in CSF and in serum is presented in tab. no. 1.
Table 1. CSF and serum characteristics
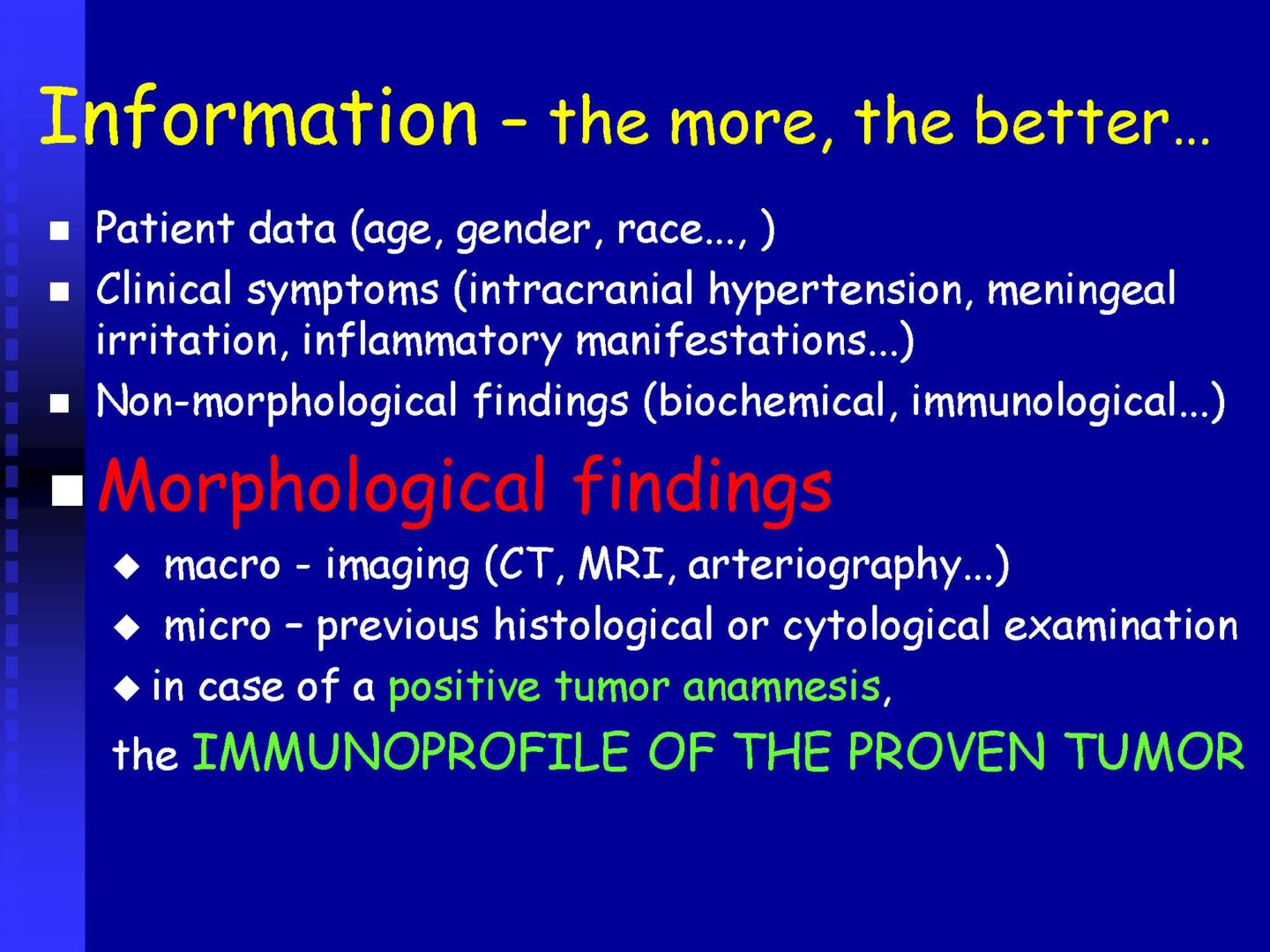
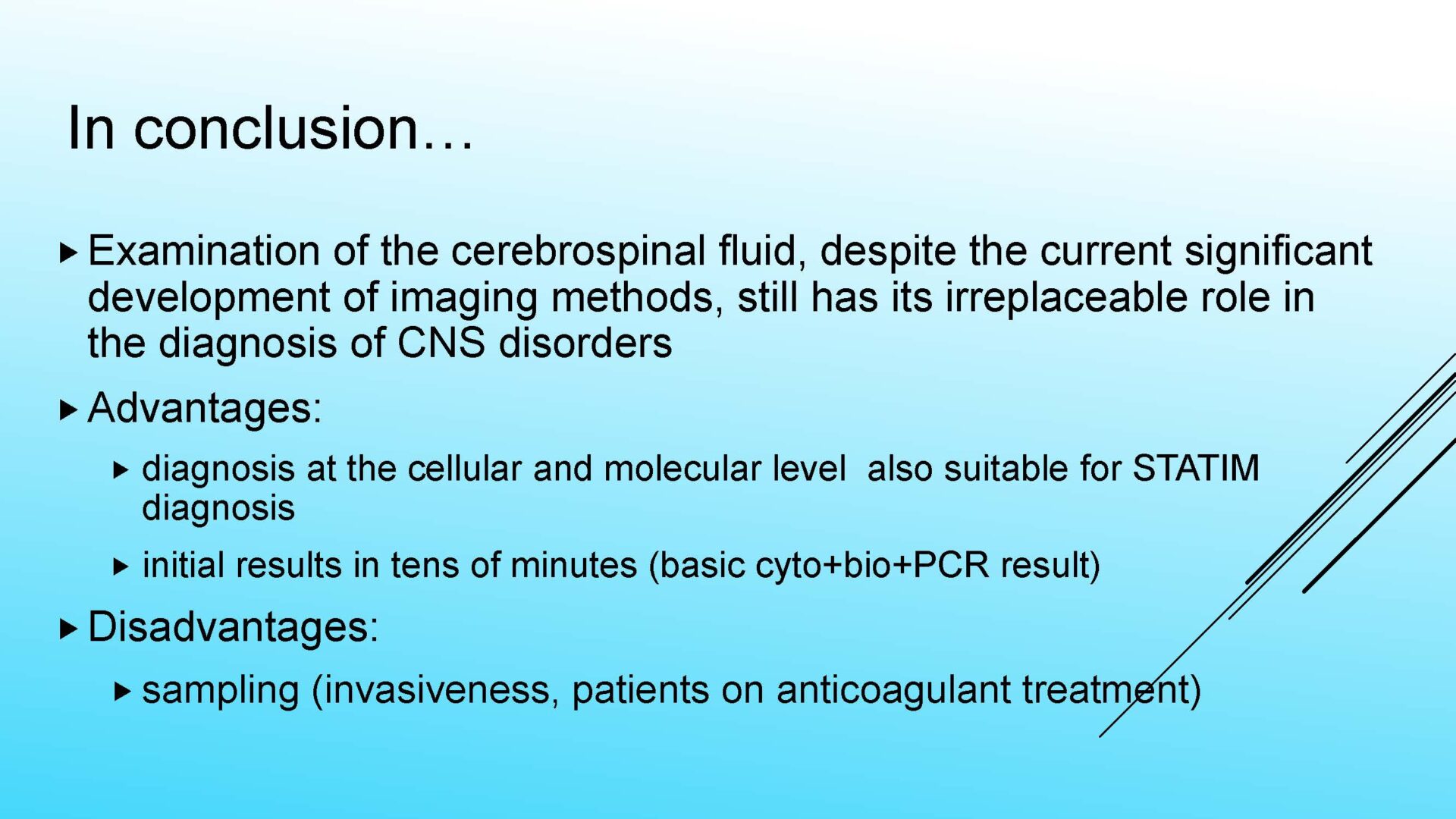
Unlike the macroscopic, or functional diagnostic information mediated by imaging, or electrophysiological examination methods, the analysis of cerebrospinal fluid is an examination at the cellular and subcellular (humoral-molecular) level.
From this point of view, even in the era of modern imaging and electrophysiological examination methods, laboratory analysis of cerebrospinal fluid is irreplaceable in the diagnosis of CNS disorders such as inflammatory, neurodegenerative diseases, tumors and, in specific situations, hemorrhagic affections.
For practical and logistical-operational reasons, laboratory examination of cerebrospinal fluid is divided into the parameters of so-called basic cerebrospinal fluid analysis and subsequently into extended examinations of special cerebrospinal fluid, which fall under the competence of specialized cerebrospinal fluid laboratories with appropriate equipment and personnel.
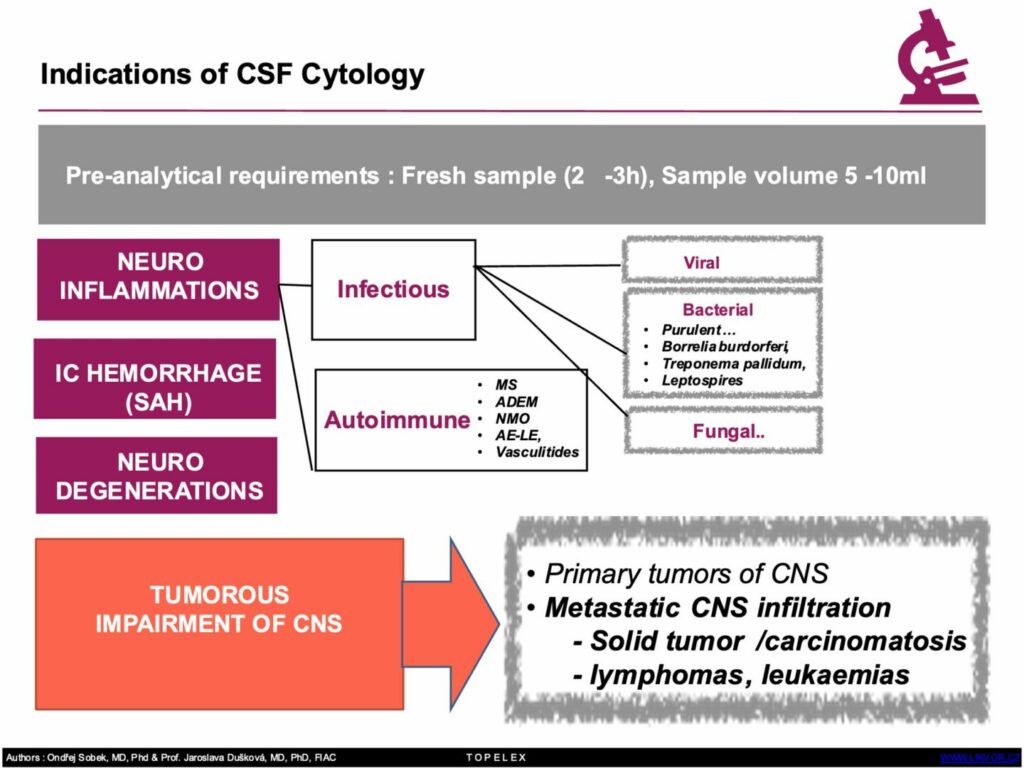
Pre-analytical requirements for CSF examination
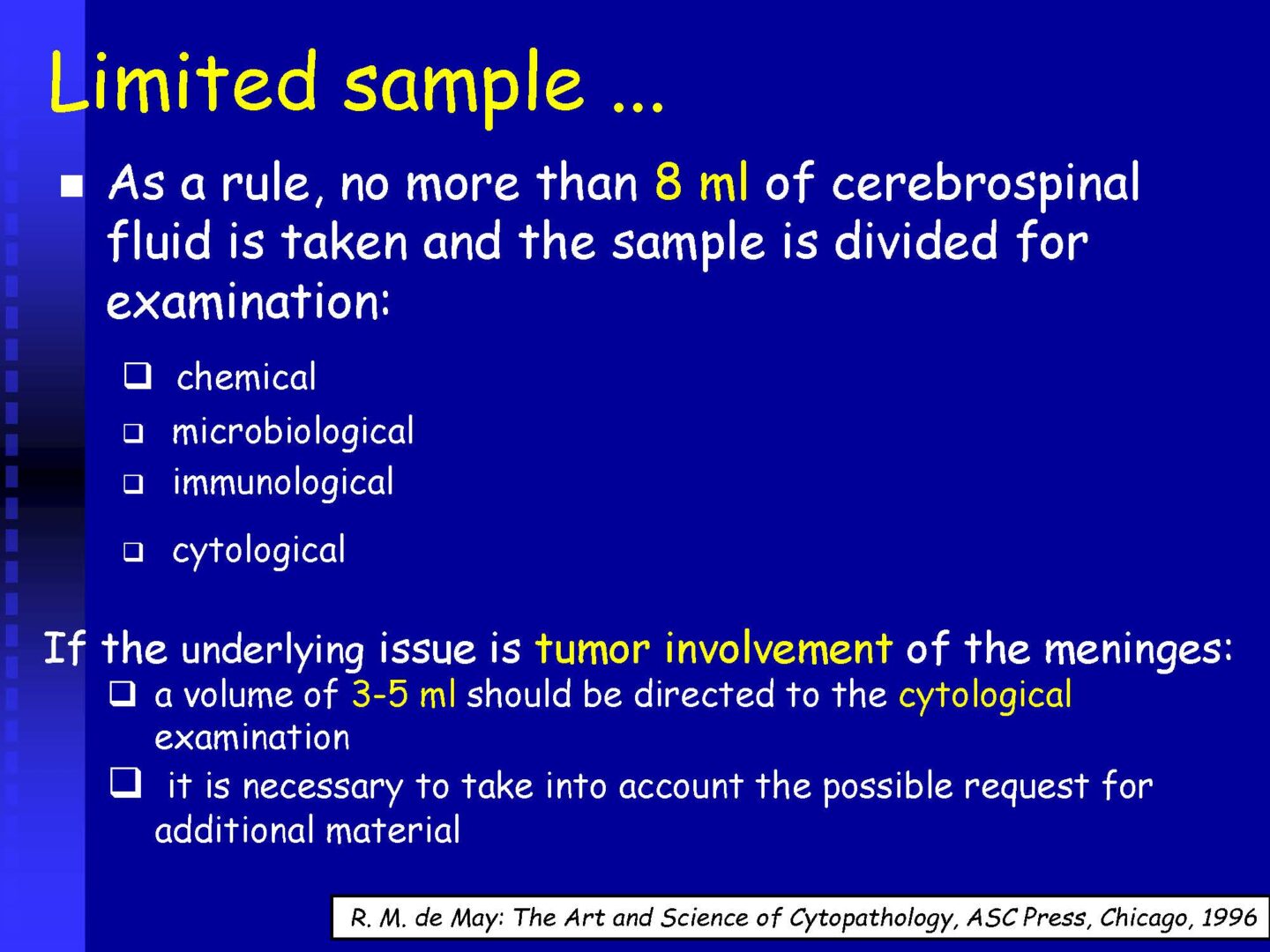
fresh sample (2-3 hours), optimum amount 5-10 ml
The recommended amount of cerebrospinal fluid is determined by the number of required examinations
Total sample volume for extended examination is 12-15 ml for an adult.
Along with the cerebrospinal fluid, it is necessary to collect and send blood for a parallel examination in the sending medical facility, so that it is possible to determine, for example, the condition of the hematoencephalic barriers, intrathecal synthesis, quotients and indices of analytes in the relationship between cerebrospinal fluid and serum.
Plastic disposable resealable tubes with rubber or plastic stoppers, without additives! (for determining markers of neurodegeneration use polypropylene tubes)
Alternatively, it can be taken into a glass tube for CSF.
Required quantity: minimally 0.8 ml of CSF (= 1 microscopic slide)
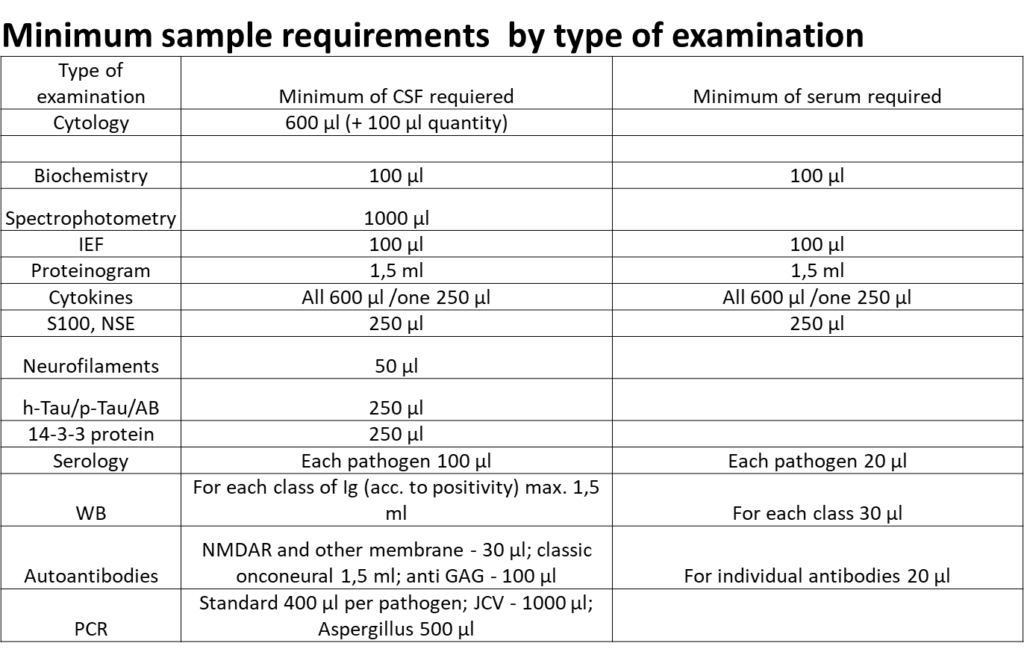
Minimum sample requirements by type of examination are summarized in table no. 2.
* If ICC or FCM testing is required, a minimum of an additional 1200 ul of CSF is needed
(depending on the cellularity of the sample).
Summary of pre-analytical phase requirements
For a complete cerebrospinal fluid examination, approx. 12-15 ml of cerebrospinal fluid and 12-15 ml of serum (coagulable blood) are needed, for cytological, PCR and serological tests min. 1 ml of sample.
Stability of the native sample: for cytology 2-3 hours (sometimes even longer – it depends on the oncotic and energetic properties of the sample).
The sample can be transported at room temperature (up to 25 degrees Celsius) – overheating or freezing of the sample must be prevented (thermobox)
Close contacts to CNS
The CSF compartment is separated from the blood compartment by a system of functional and anatomical barriers
Specific characteristics
small volume (150 ml)
hypo-oncotic
oligocellular under physiological conditions
Indications for examination of cerebrospinal fluid
Differential diagnosis of pathological processes of the brain, spinal cord, meninges and systemic diseases
Inflammation
Infectious
bacterial
purulent
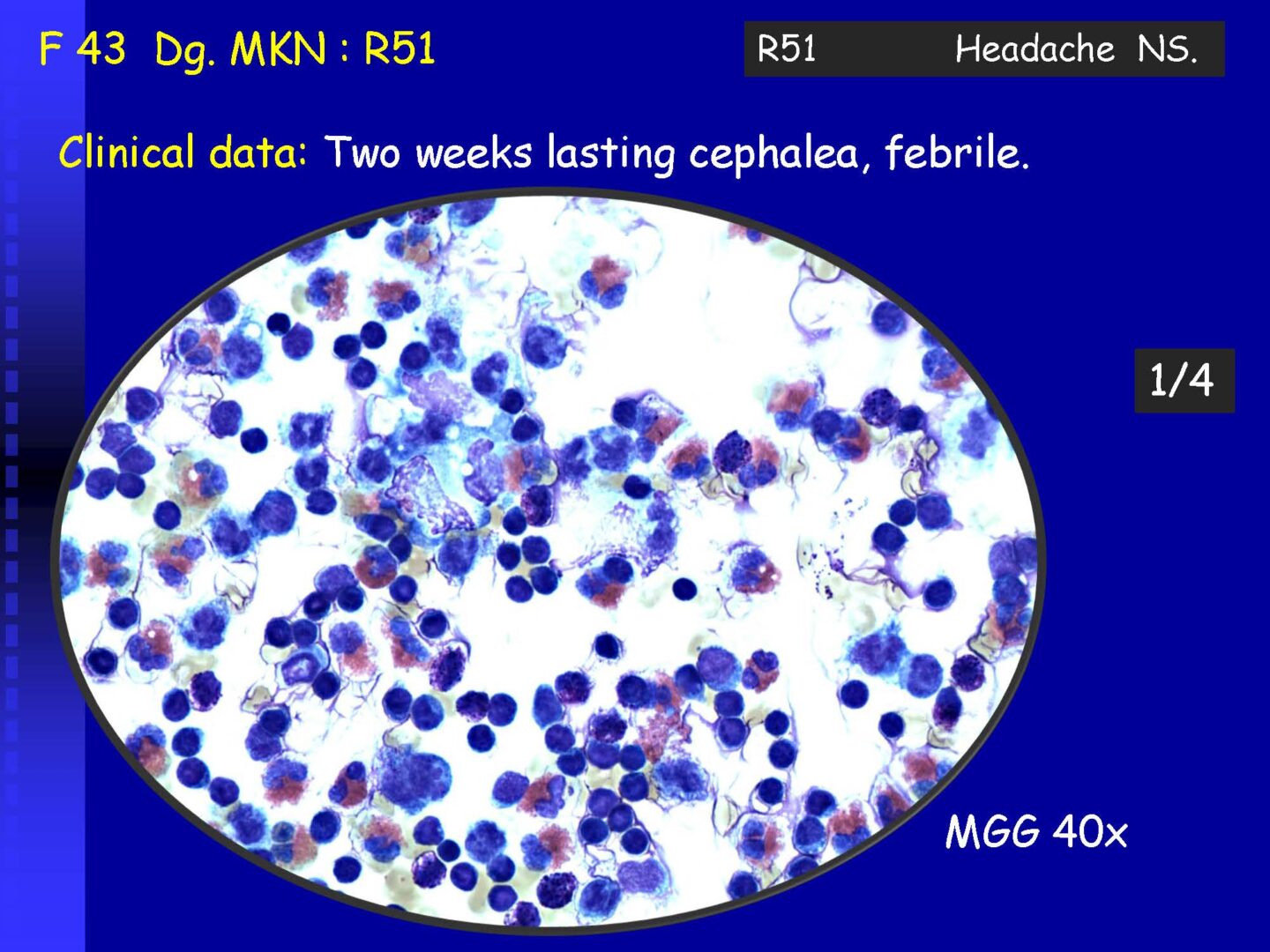
serous: Borrelia, Treponema, Leptospira…
viral
mycotic
parasitic
autoimmune
Multiple Sclerosis – MS
Acute Demelinating Encephalomyelitis – ADEM
NeuroMyelitis Optica Spectrum Disorders- NMOSD, Myelin Oligodendrocyte Glycoprotein Antibody Disease-MOGAD
Paraneoplastic neurological syndromes (PNS)
Autoimmune Encephalitis, Limbic encephalitis – AE, LE
Vasculitides of CNS
Acute / Chronic Inflammatory Demyelinating Polyradiculoneuropathy – AIDP/ CIDP
Intracranial bleeding- mainly SubArachnoidal Haemorrhage (SAH)
Neurodegenerations
Dementias (Alzheimer Disease – AD)
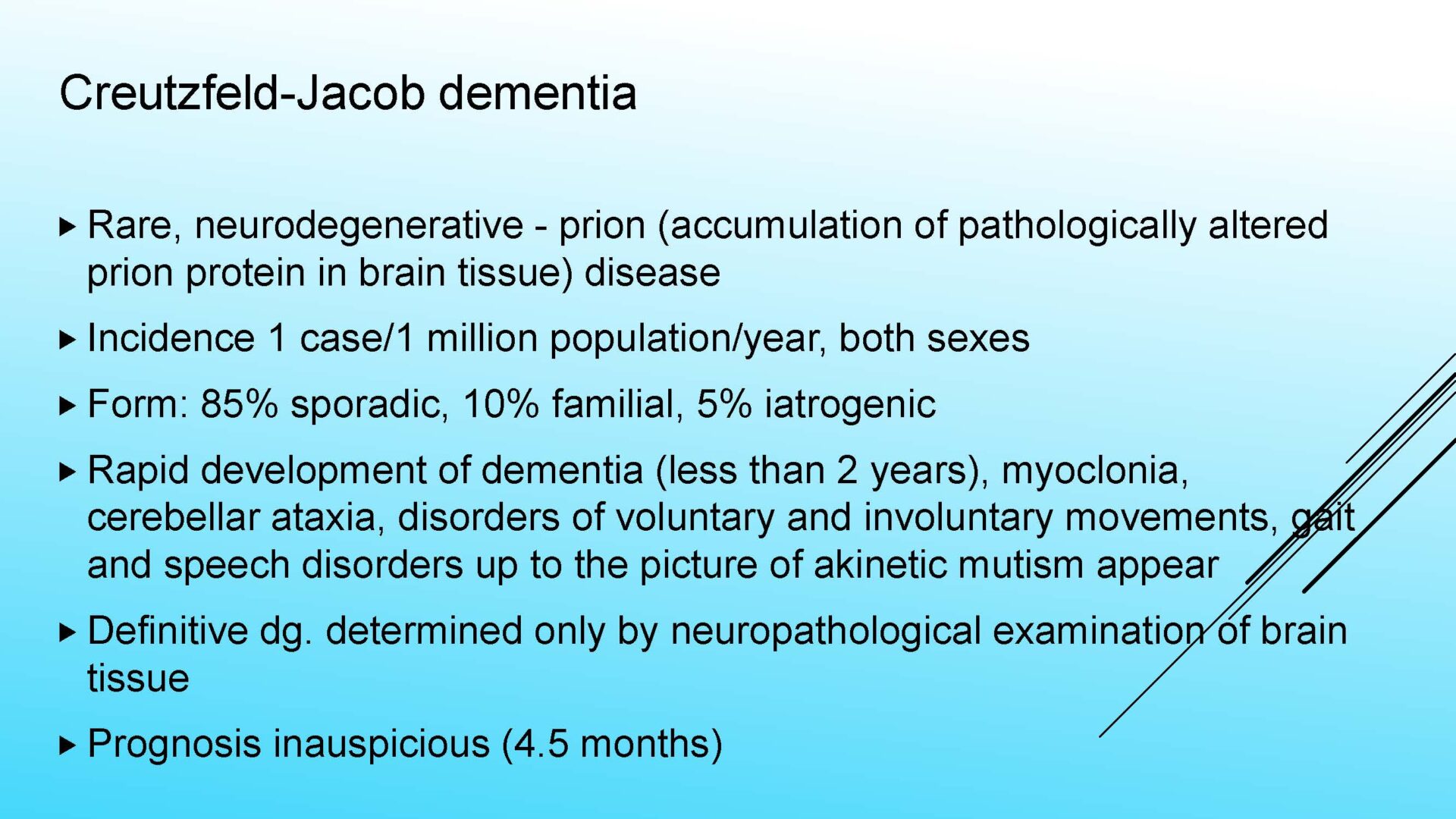
Prionopathies (Creutzfeldt-Jakob Disease – CJD )
Motor Neuron Disease (Amyotrophic lateral sclerosis – ALS)
Neoplasms
primary
secondary
solid: carcinomatoses/ melanoma/ germ cell/ sarcomas
lymphomas and leukemias
Table 3. Summary of CSF cytology indications
Conception of CSF diagnostics
Parameters of basic CSF examination – including cytology
acute availability – in the case of an urgent laboratory testing regime
basic rapid identification of the pathology process
indication of subsequent specialized tests
Specialized tests
detailed identification of the pathology process
laboratory-clinical interpretation
Contraindications of lumbar puncture
intracranial hypertension with risk of occipital herniation(i.e. especially during expansive intracranial processes)
hemocoagulation disorders (threatening by bleeding into the spinal canal after puncture…)
florid skin infection at the injection site (introduction of infection)
Spinal tap
CSF is routinely obtained from the spinal canal by lumbar puncture in the area of L3/4, L4/5, or L5/S1.
The position of the patient during sampling has an effect on the measured pressure values in the CSF space – in the supine position it is up to approx. 20 cm of water column (15 mm Hg), the values can be up to double for a person sitting.
To assess the patency of the cerebrospinal fluid tracts, the so-called Queckenstedt test (when the jugular veins are compressed causes an increase in cerebrospinal fluid pressure) and the Stoockey test (an increase in abdominal pressure also leads to a rise in cerebrospinal fluid pressure) is used.
If possible, the cerebrospinal fluid is collected into 2-3 test tubes (gradual dilution of portions of cerebrospinal fluid with a small artificial blood admixture) in a sufficient amount, i.e. usually 10-15ml for an adult patient.
Sterile collection material – tubes without anti-clotting additives!
An atraumatic needle minimizes post-puncture leakage of cerebrospinal fluid, causing cerebrospinal fluid hypotension and post-puncture complications (headache, dizziness, nausea)
If biochemical, microbiological or neuroimmunological tests are required in addition to cytology, it is necessary to take whole blood (again 10-15 ml) in parallel (i.e. within about 30 min) with cerebrospinal fluid collection, as it is necessary to know the parallel blood concentrations of analytes for calculation and interpretation of their so-called intrathecal synthesis.
Suboccipital puncture
A less common and now practically no longer used routine method of collecting cerebrospinal fluid is a suboccipital puncture of the cisterna magna performed below the occipital axis medially through the atlanto-occipital membrane
The risk is impingement of aberrantly running vessels (a. vertebralis, a. cerebelli inf. post.) with potentially fatal intracranial bleeding.
Ventricular puncture
Extraction of cerebrospinal fluid directly from the cerebral ventricles (ventricular puncture) is already classified as a neurosurgical procedure.
The basic examination of the cerebrospinal fluid, which should be optimally available locally and in time, includes
quantitative cytology ( “cell count” -determination of the number of nuclear elements and erythrocytes)
qualitative cytology with the preparation of a permanent cytological preparation in basic staining (usually May-Grünwald / Giemsa-Romanowski)
determination of total protein , glucose, lactate and spectrophotometric examination.
This panel of basic laboratory parameters enables us to detect with relatively high sensitivity the basic types of pathological processes in the cerebrospinal fluid, i.e. the CNS compartment, and further to determine the basic direction of differential diagnostic direction with the selection of the appropriate spectrum of subsequent laboratory examinations within the parameters of the so-called extended special cerebrospinal examination – see chapter Extended CSF and neuroimmunological examination.
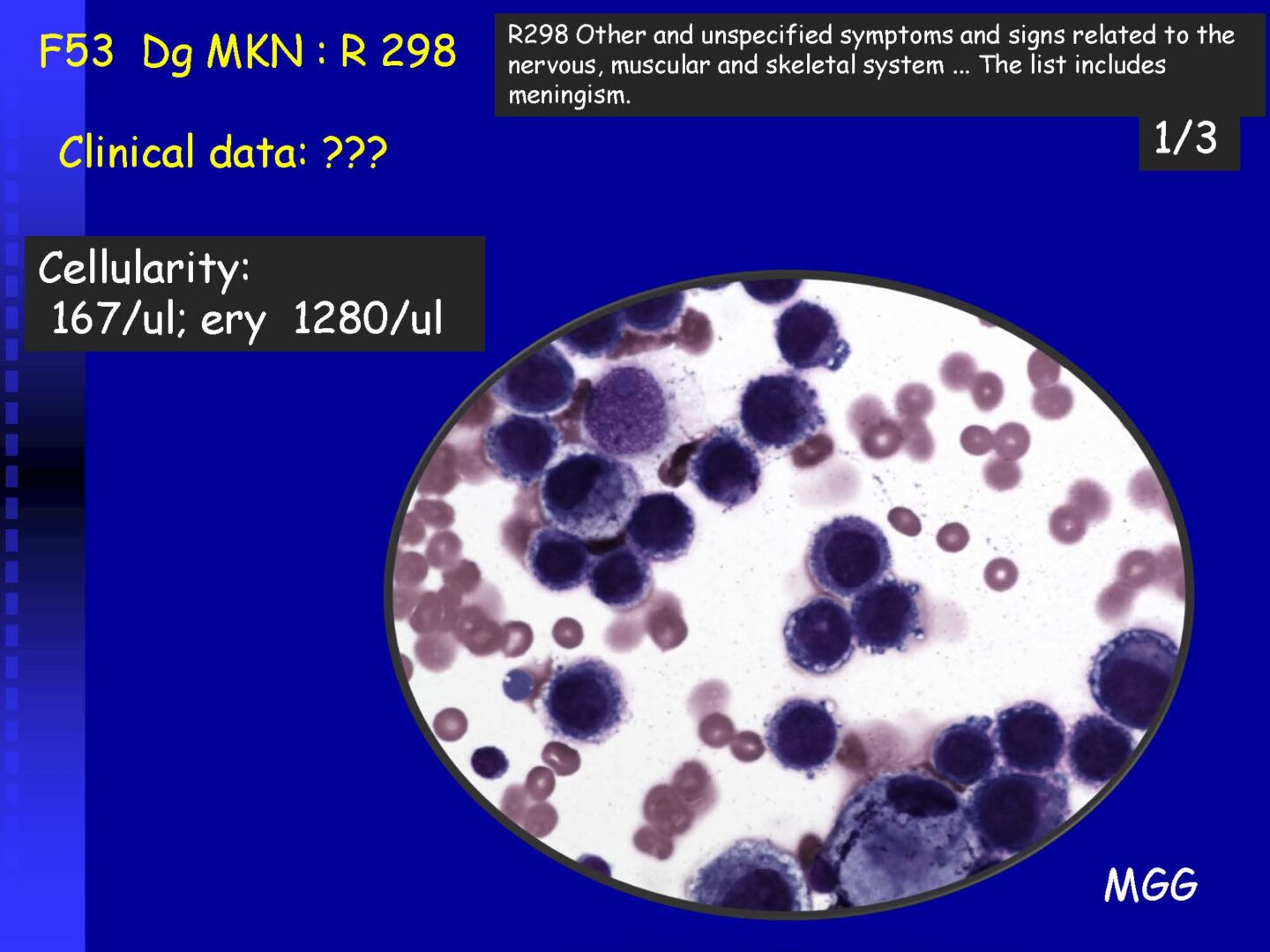
Cellularity
does not exceed 4 elements /1 ul (10/ 1 ul in newborns)
of this amount, 65-80% are made up of small lymphocytes, the rest are represented by monocytes
activated forms are not present (plasma cells, macrophages with manifestations of phagocytic activity…).
Contaminants
erythrocytes
neutrophils (NG) *
meningothelial cells
cells of choroid plexus
ependyma
chondrocytes
*The tolerated amount of NG is calculated from blood contamination (approx. 1 NG per 700 erythrocytes)
Under physiological circumstances, lymphocytes and monocytes are found in the cerebrospinal fluid (in a ratio of roughly 60-80% : 20-40%), and these are their resting stages.
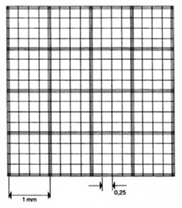
The cellularity of the CSF in a healthy individual is low, not exceeding 10 nucleated elements in 3 microliters as counted in the Fuchs – Rosenthal counting chamber (Fig.4A,4B), i.e. 4 elements per microliter. The counting procedure (will be described in more details) starts with the evaluation of erytrocytes that are not a physiological component but frequently present in small amounts as a contaminating result of the puncture.
Under physiological conditions, there are no activated cellular elements, i.e. we do not find plasmacytes in the lymphocyte row and in monocytic line of macrophages (i.e. no phagocytic activity is present).
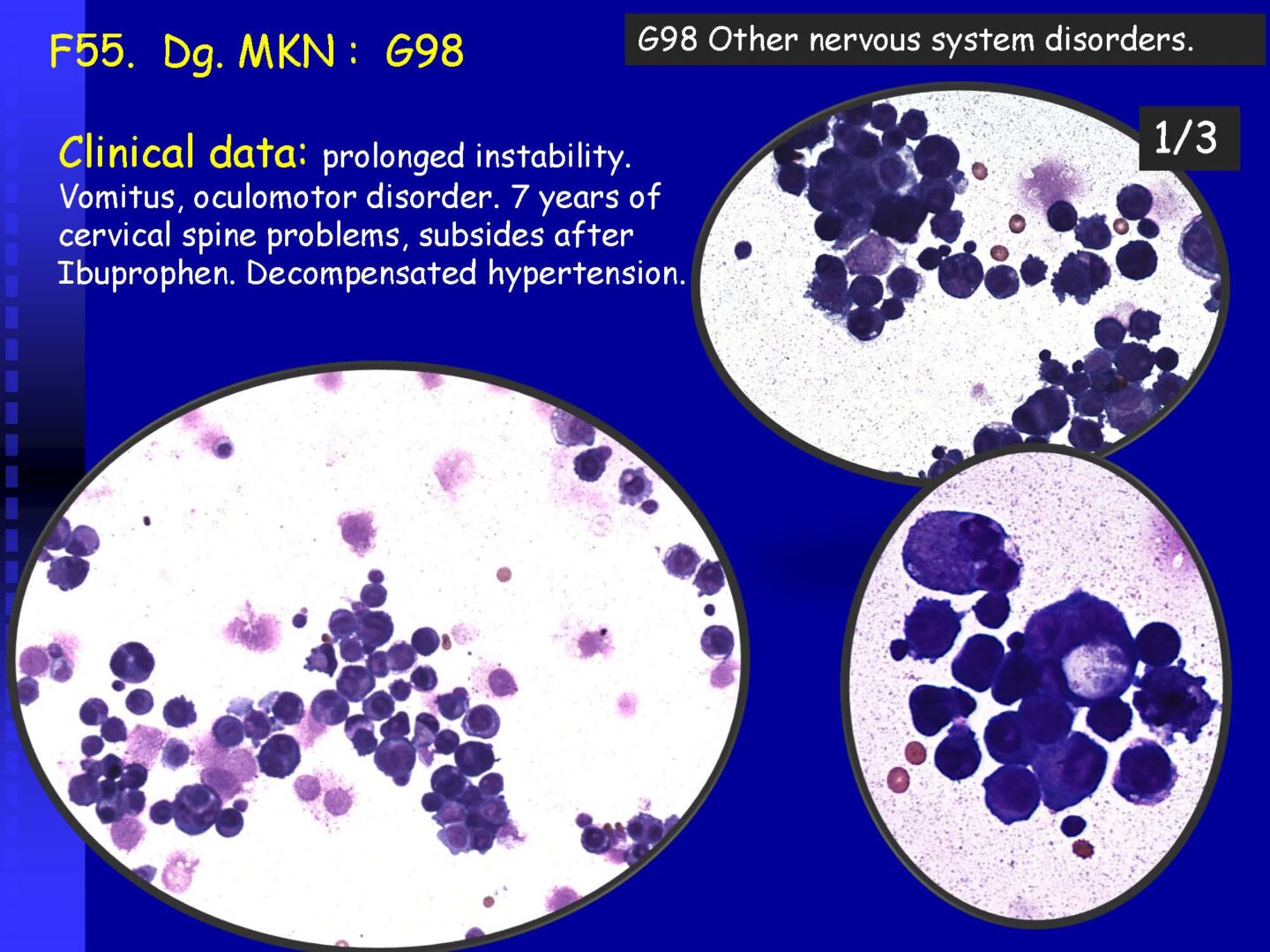
We refer to an increased number of elements as pleocytosis.
Depending on the predominant type of cells we speak of
lymphocytic
monocytic
granulocytic (with a predominance of granulocytic leukocytes) or
tumorous pleocytosis (when tumor elements are found).
Even with a normal (exceptionally even reduced) number of cells in the cerebrospinal fluid, their composition can be pathological, for example, there are activated elements such as plasma cells or macrophages.
We refer to this patological condition with the general term oligocytosis, analogously according to the predominant cell line such as
lymphocytic
monocytic
granulocytic or
tumorous oligocytosis
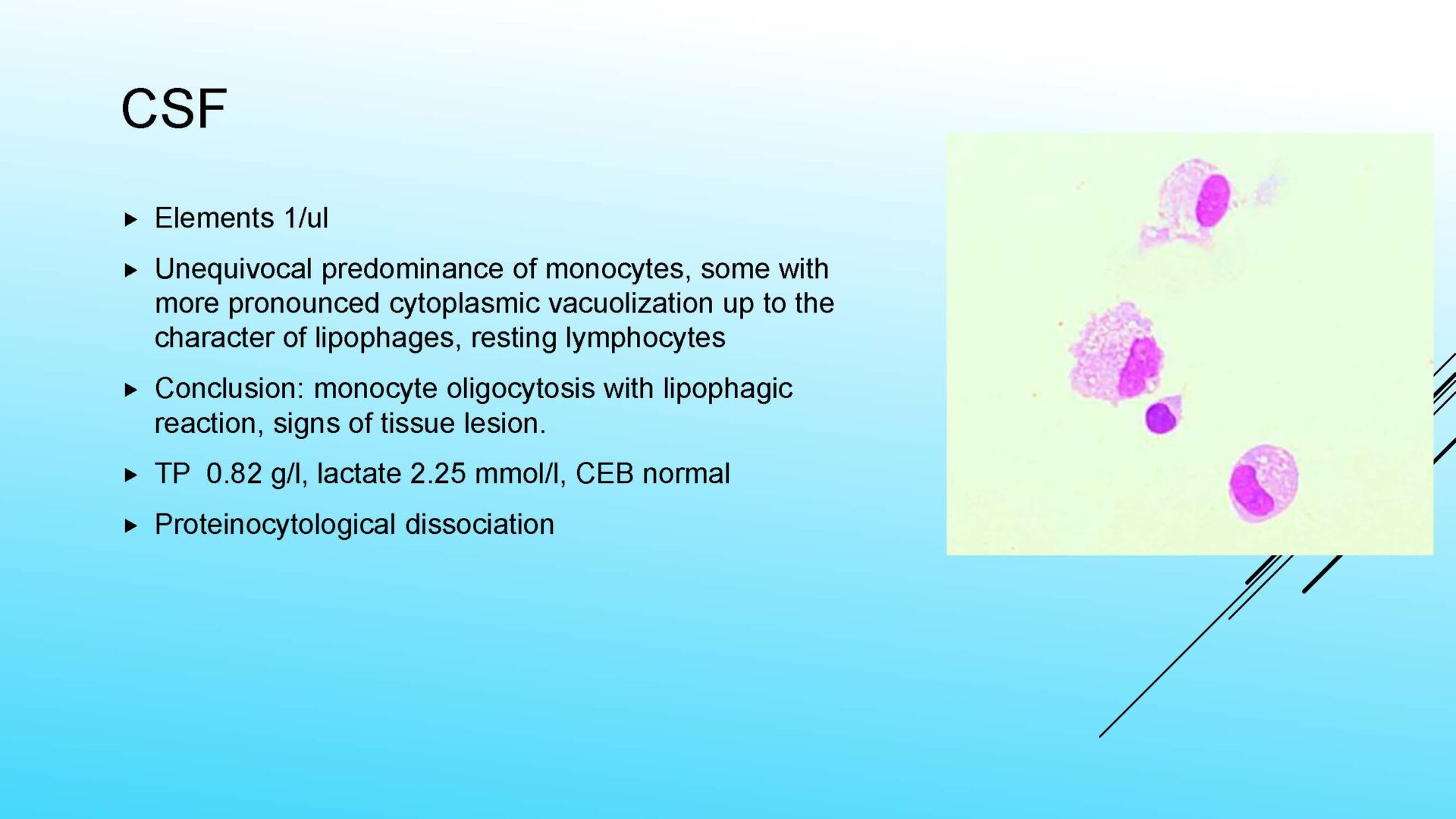
From a diagnostic point of view, a closer classification of the macrophagic response within the cytological picture is important, especially identification of a specific substrate of phagocytosis:
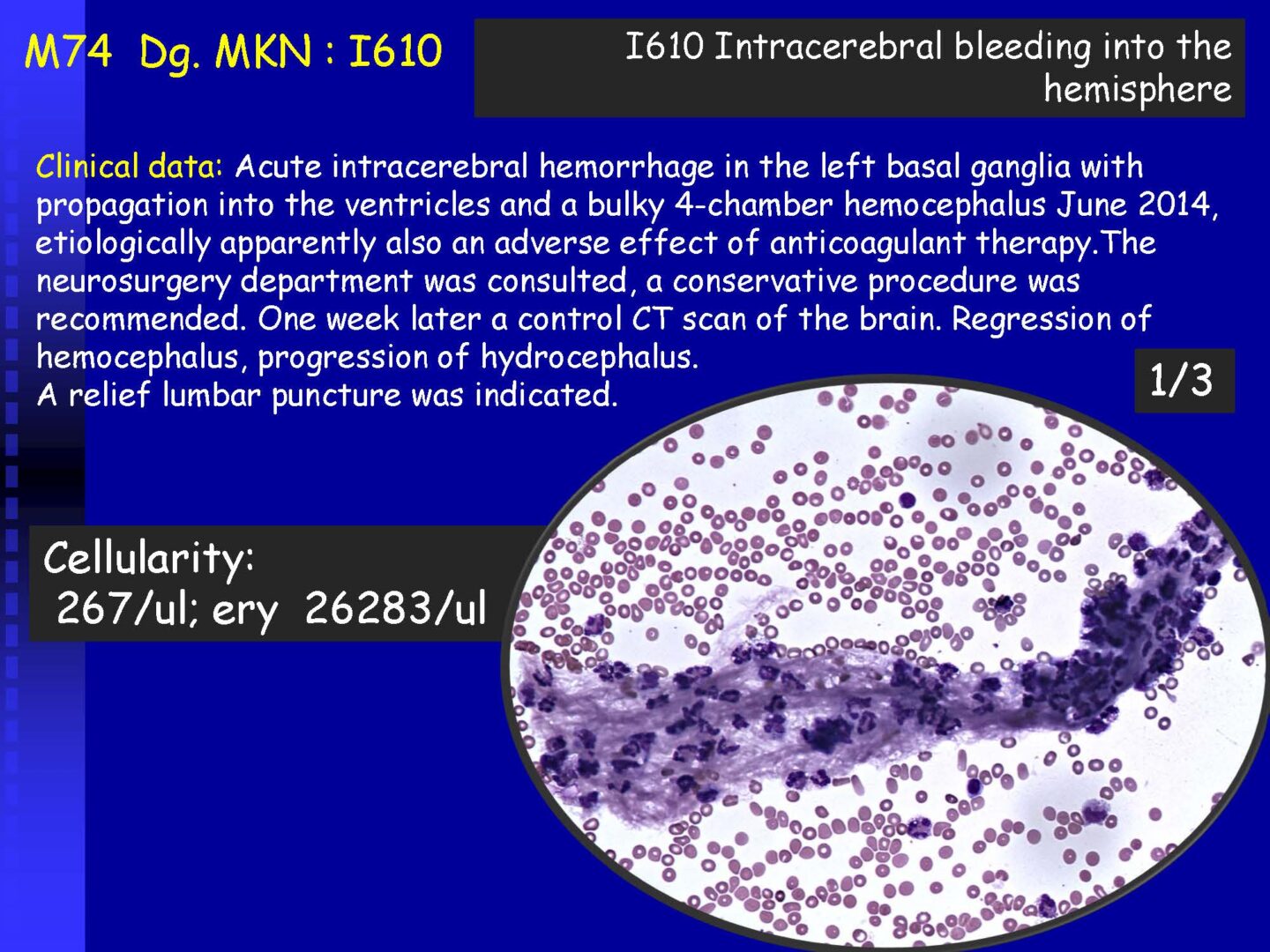
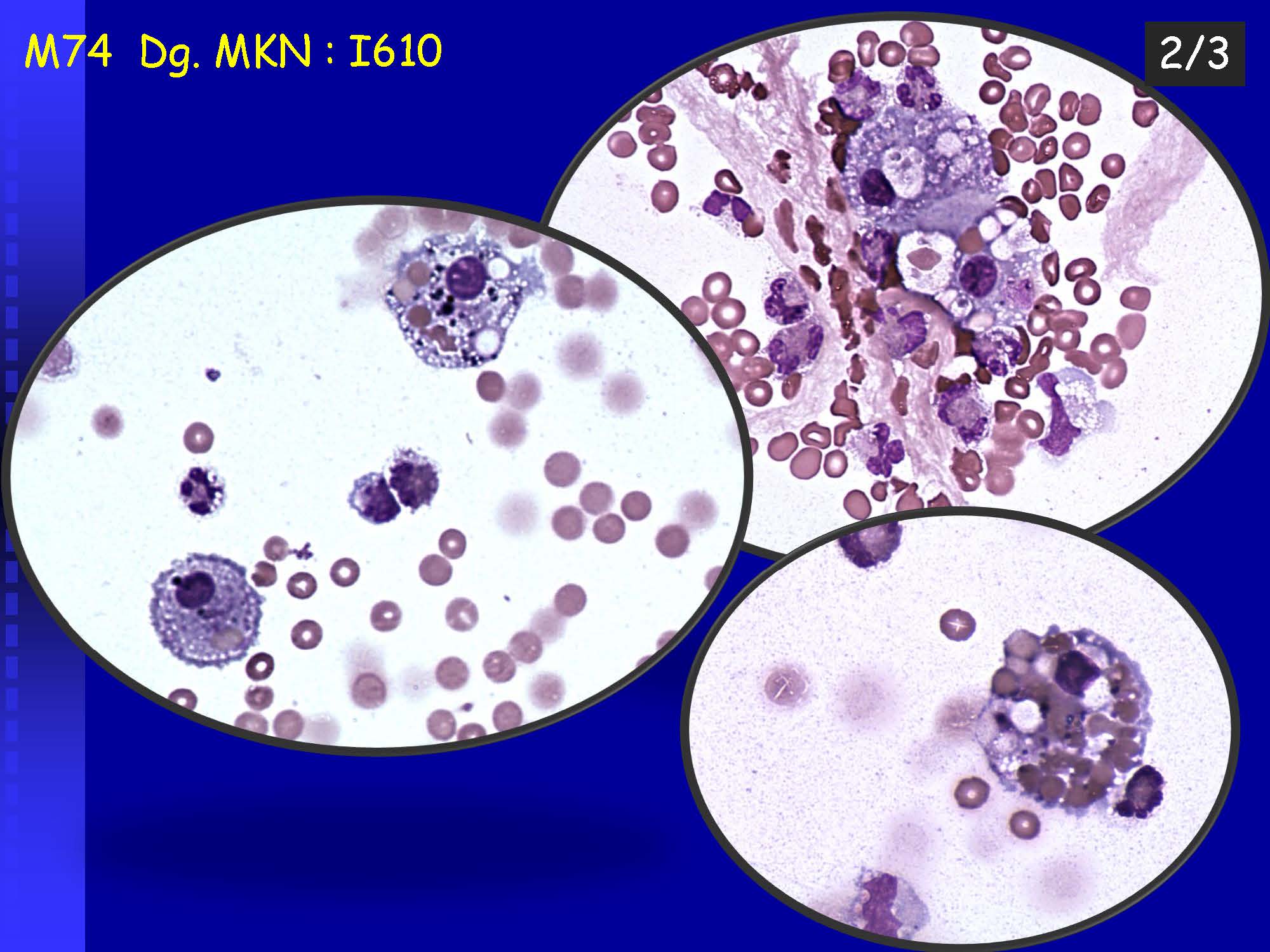
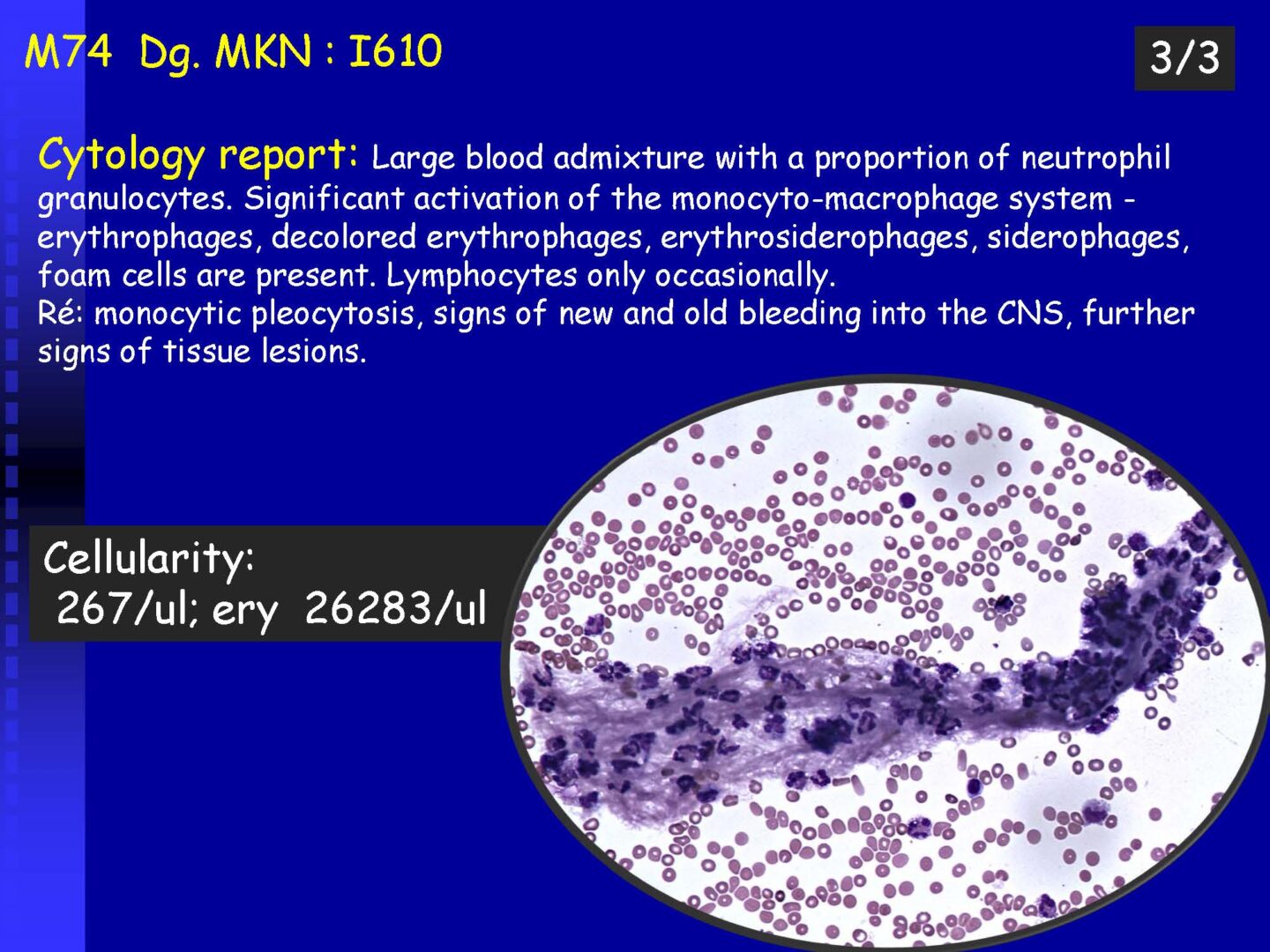
• Erythrophages – phagocytosed erythrocytes, in fresh bleeding (after 4 – 6 hours)
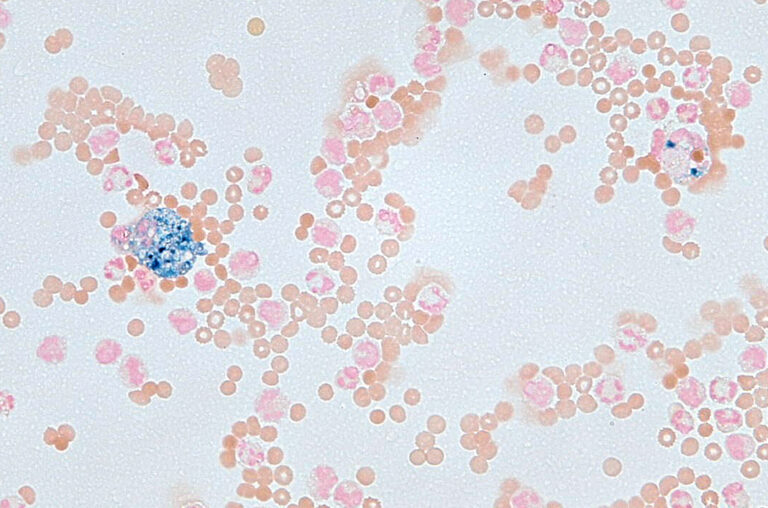
• Siderophages – phagocytosed hemosiderin, a degradation product of hemoglobin (4th-5th day after bleeding, in the early phase special iron staining must be used)
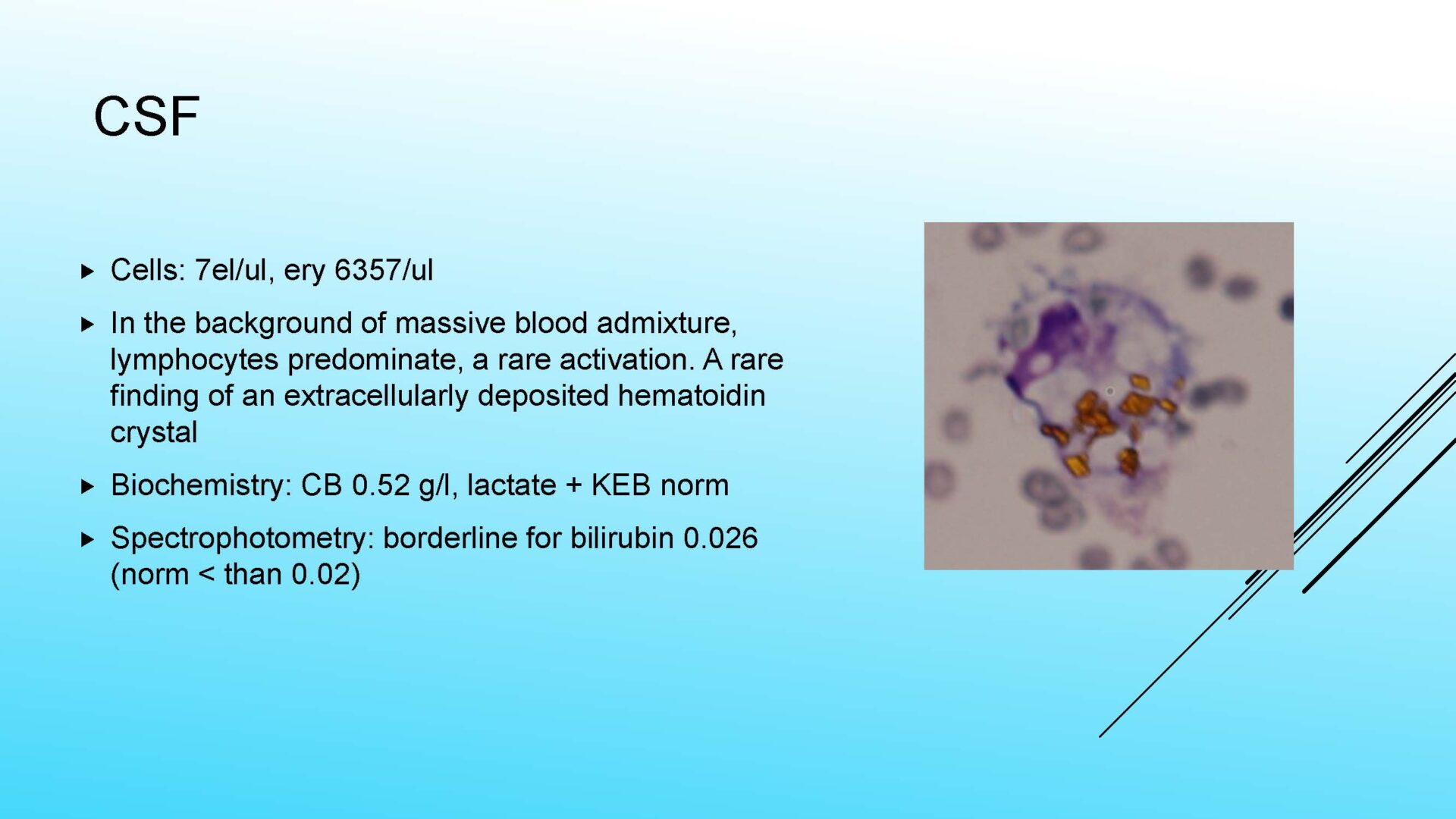
• Macrophages containing hematoidin – crystalline bilirubin, in the late phase after bleeding (from about the 13th day). It can be present extracelularly as well.
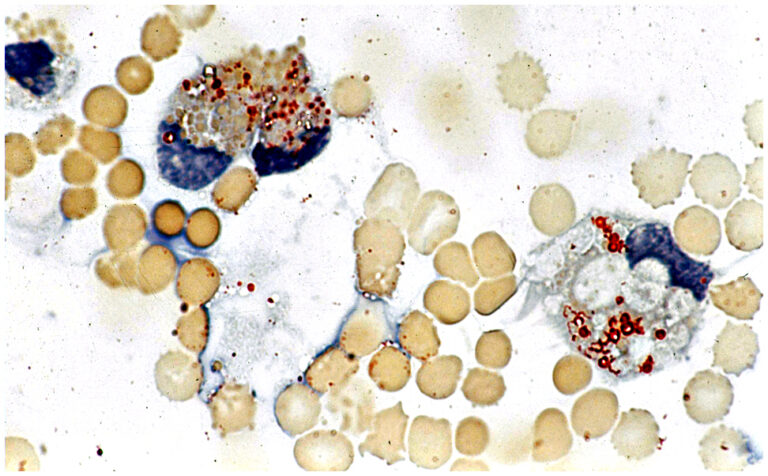
• Lipophages – so-called foamy cells in basic staining (special staining for lipids must be used for confirmation – e.g. ORO) – a manifestation of the cleaning reaction after structural damage to nervous tissue – a sensitive but etiologically non-specific finding, occurs in necrotizing neuroinfections, trauma, ischemia, bleeding, tumor infiltrations…
• Lymphophages – phagocytosed lymphocytes, in the late stage of serous inflammations
• Leukophages – phagocytosed granulocytes, in the late stage of purulent inflammation
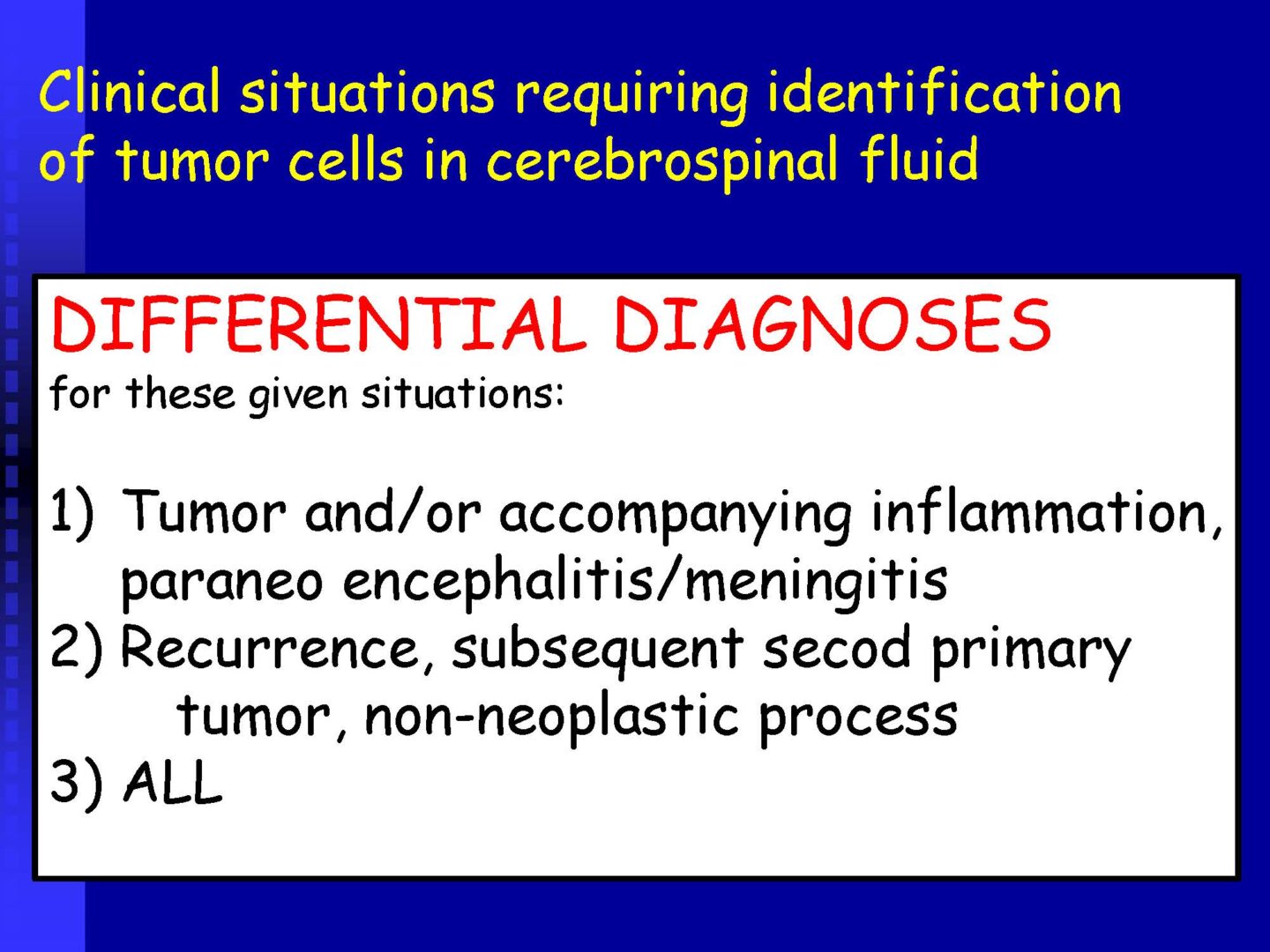
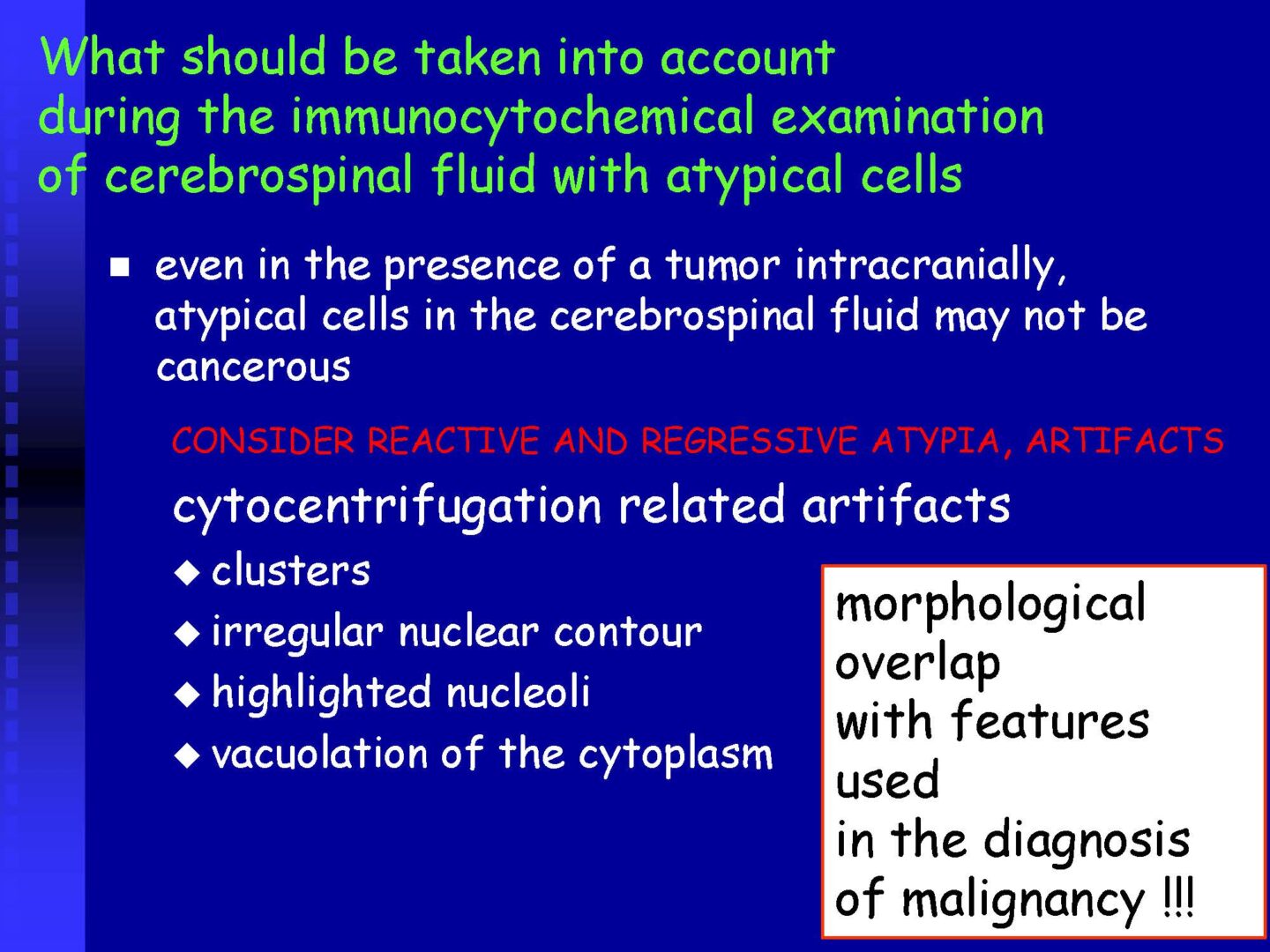
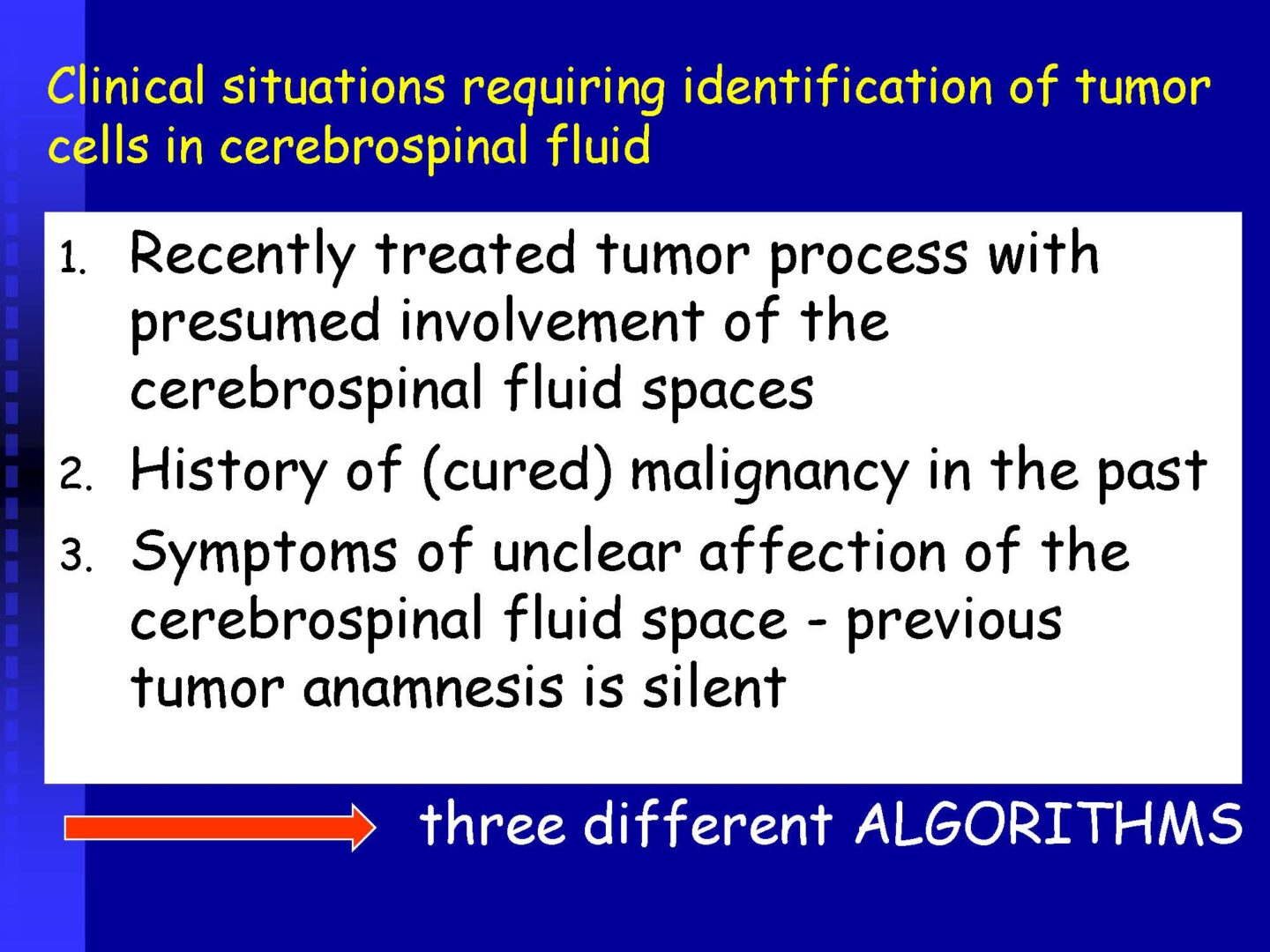
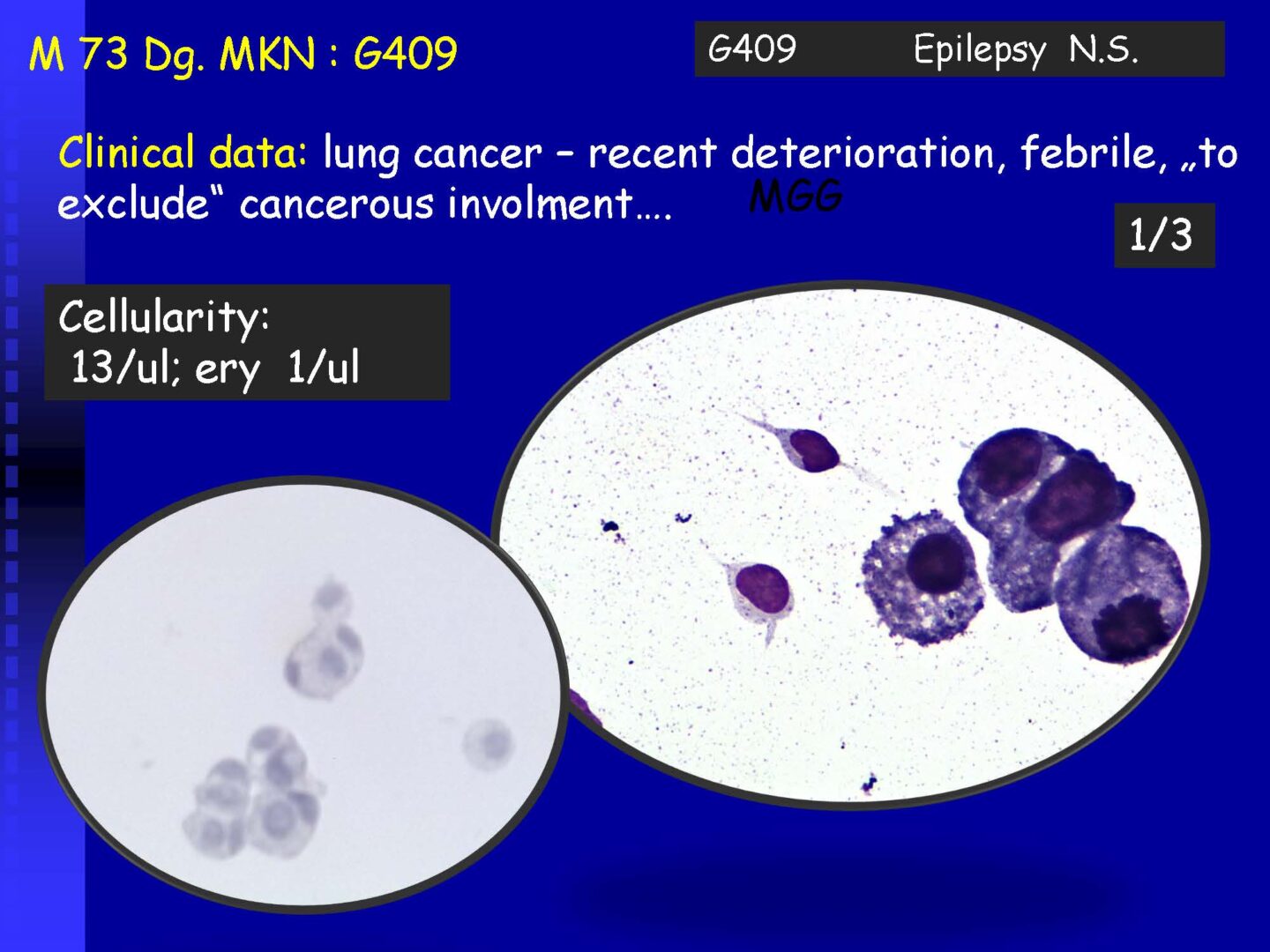
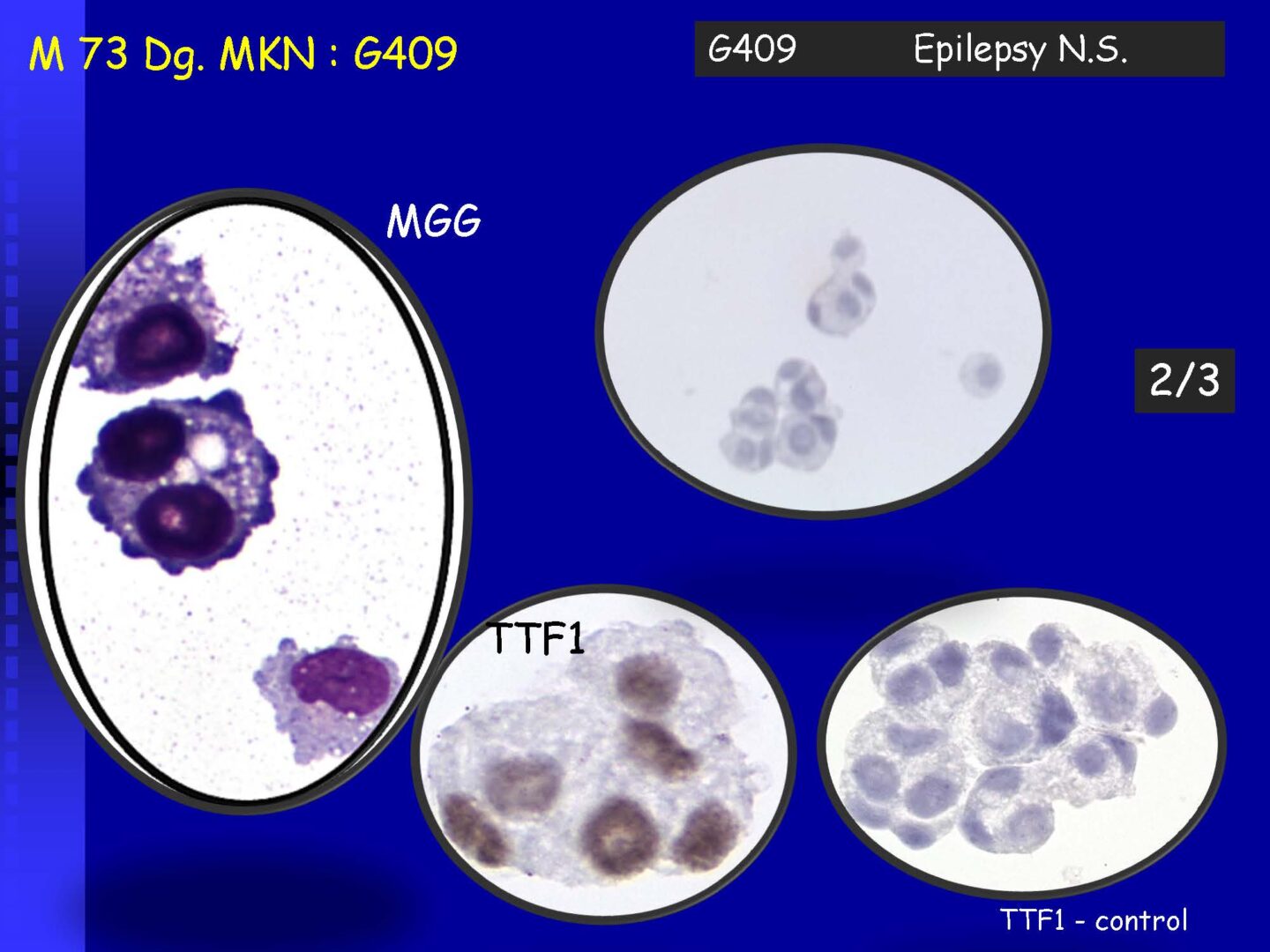
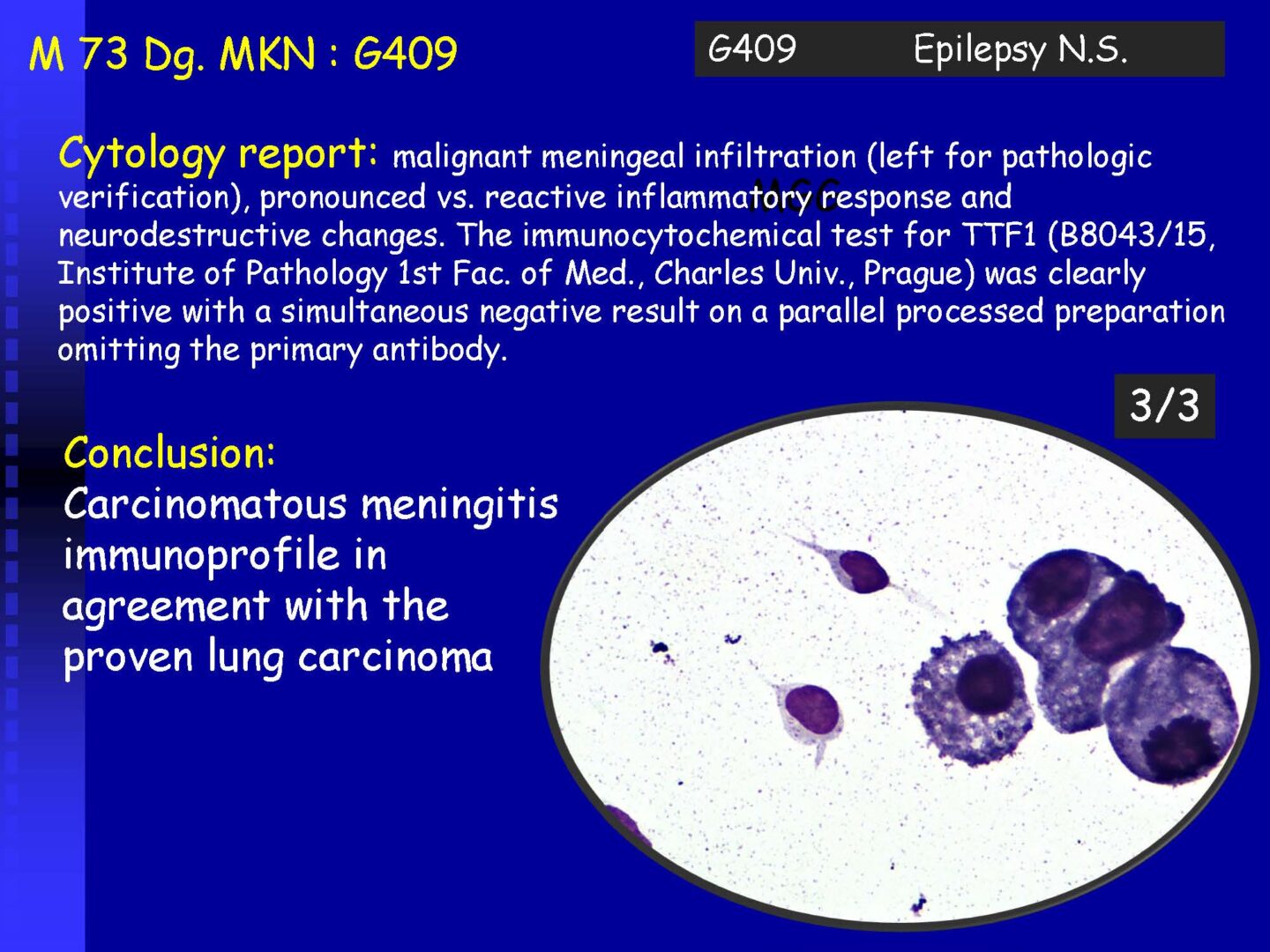
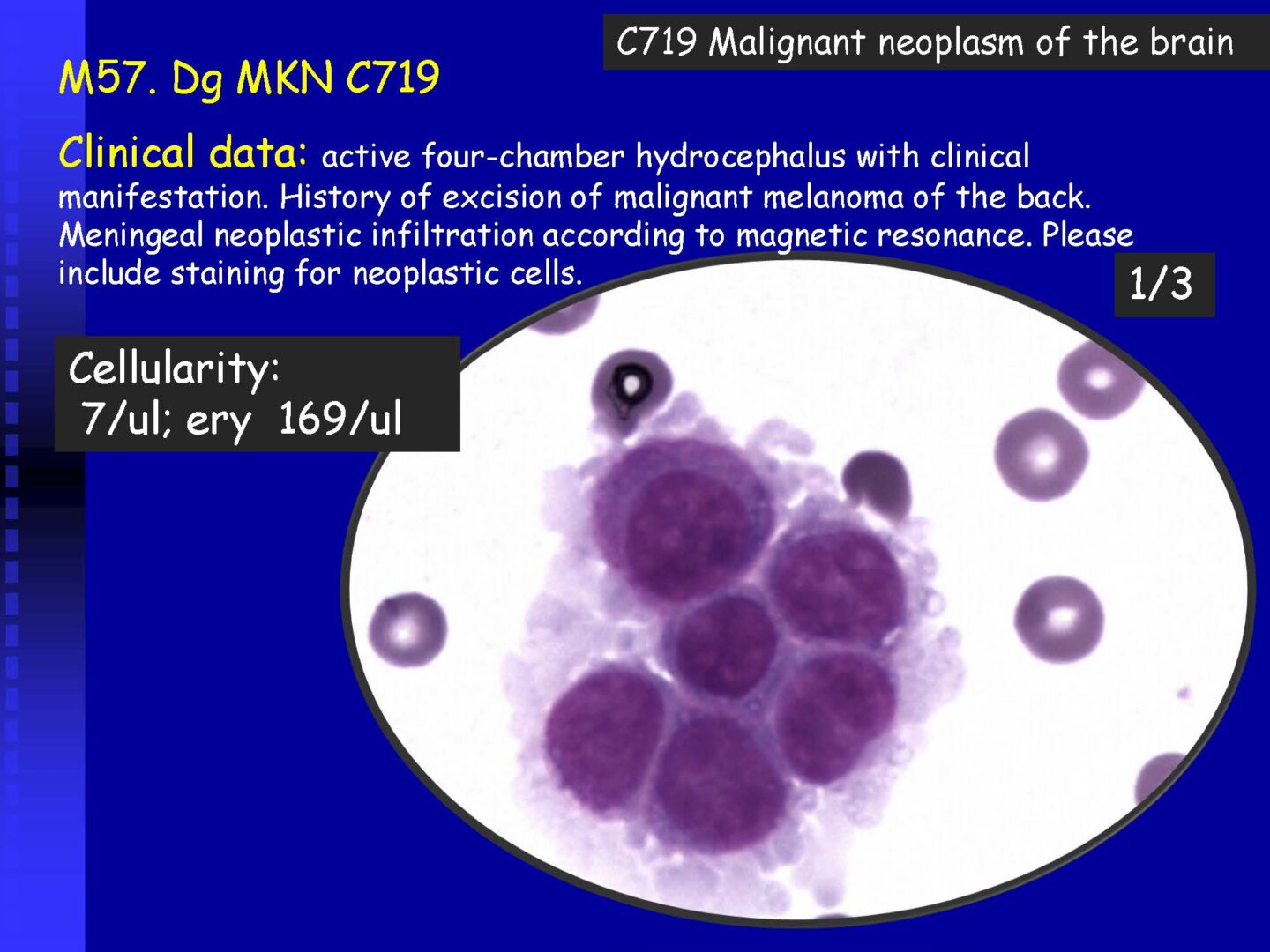
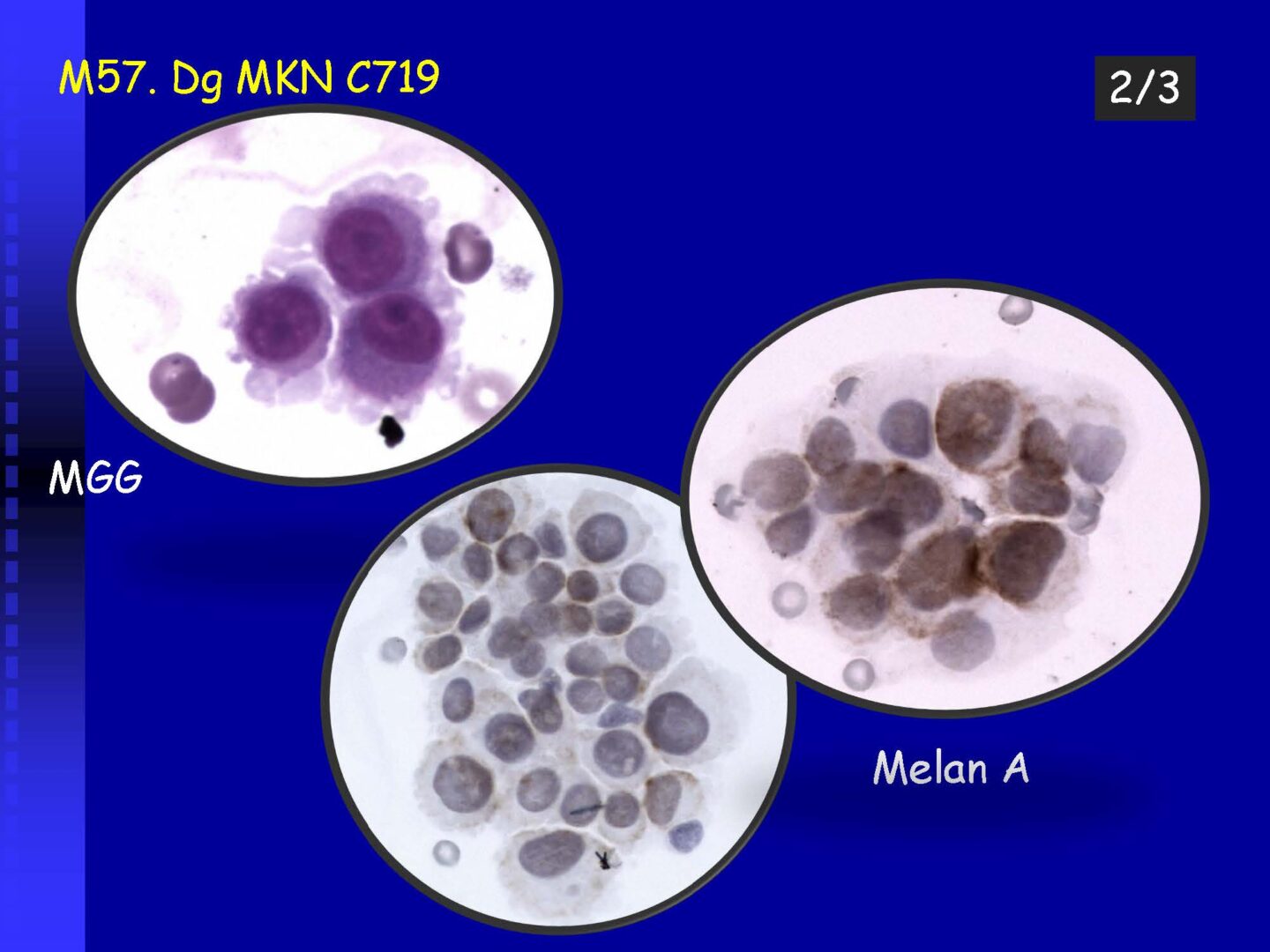
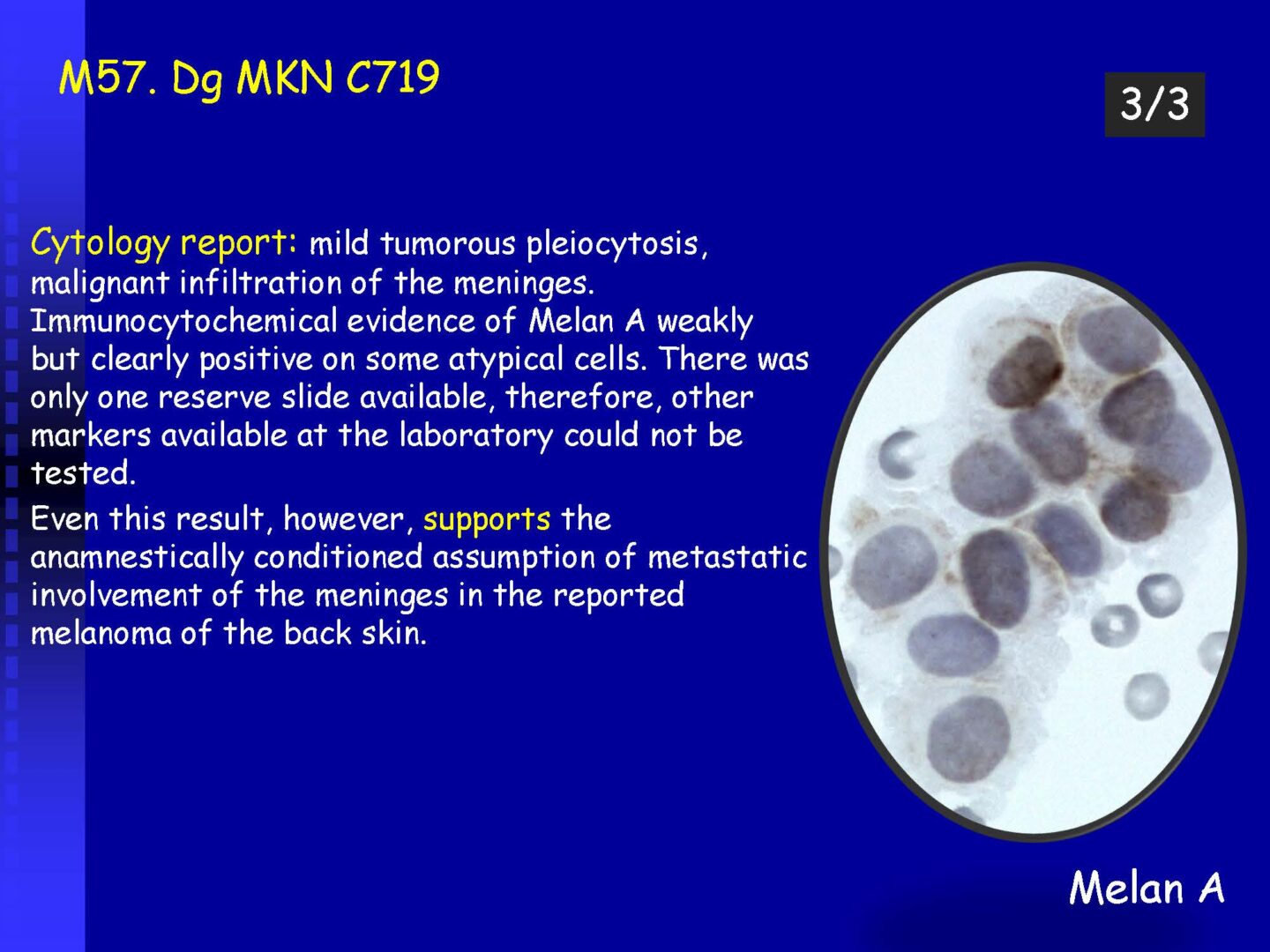
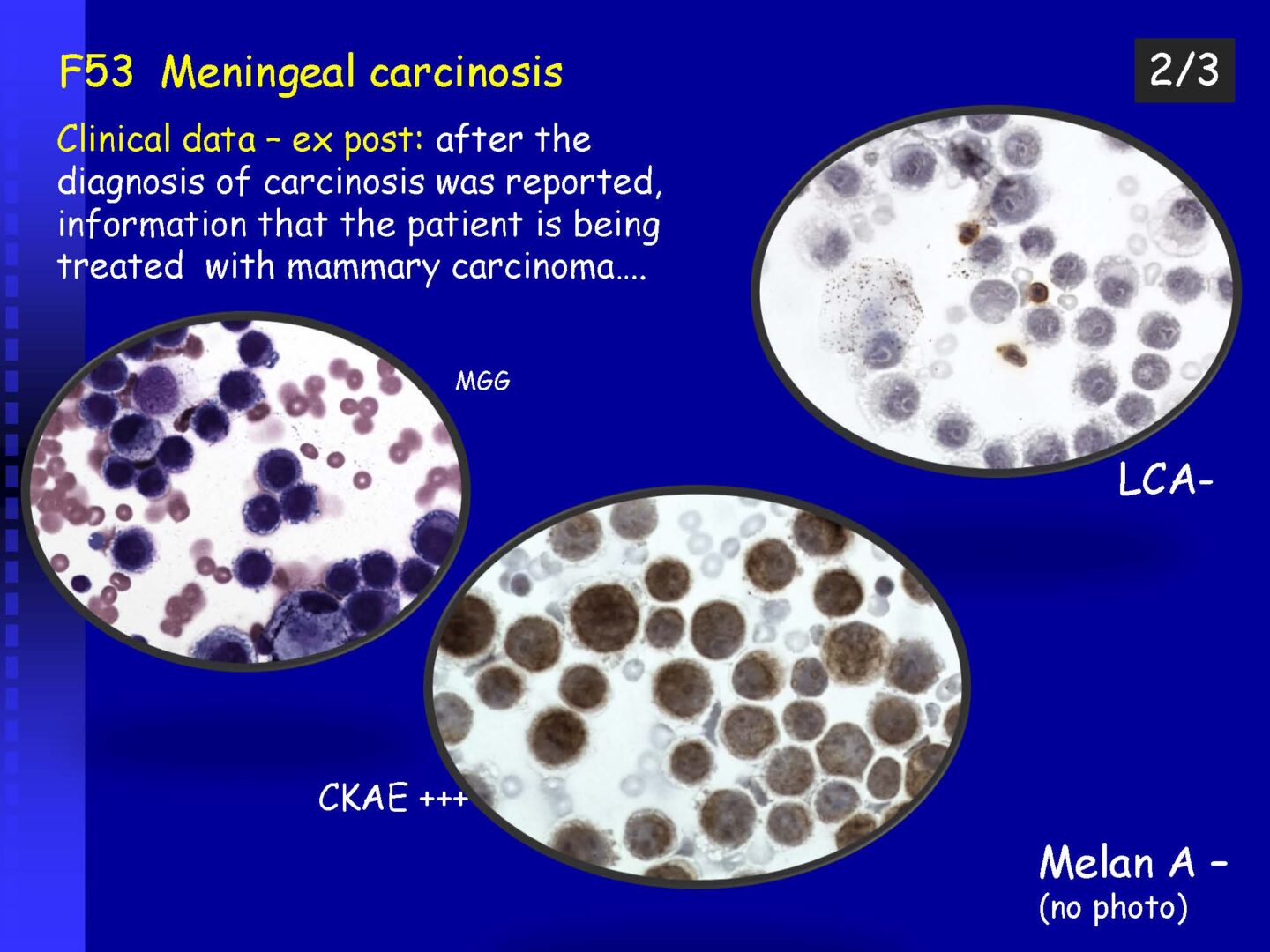
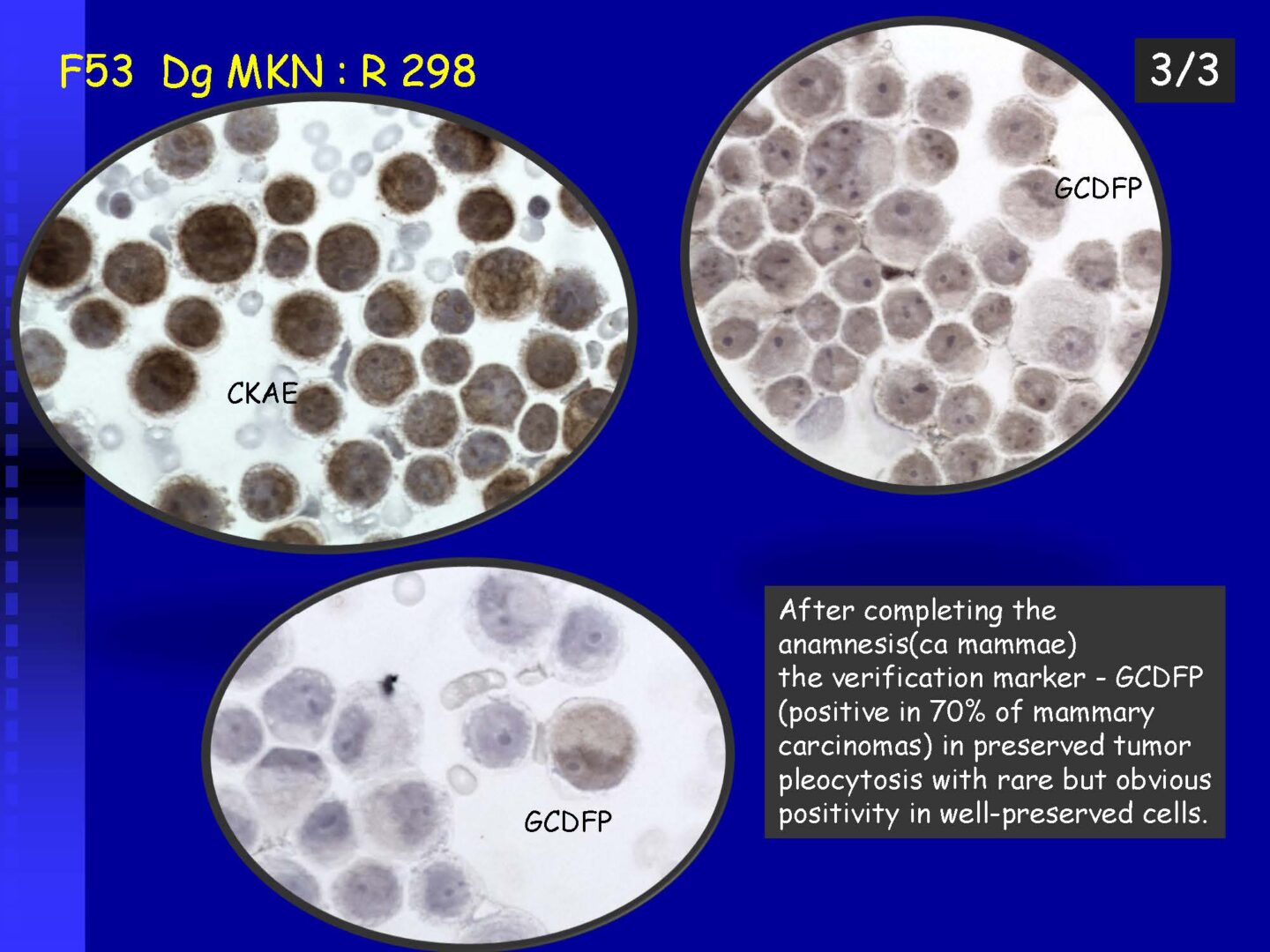
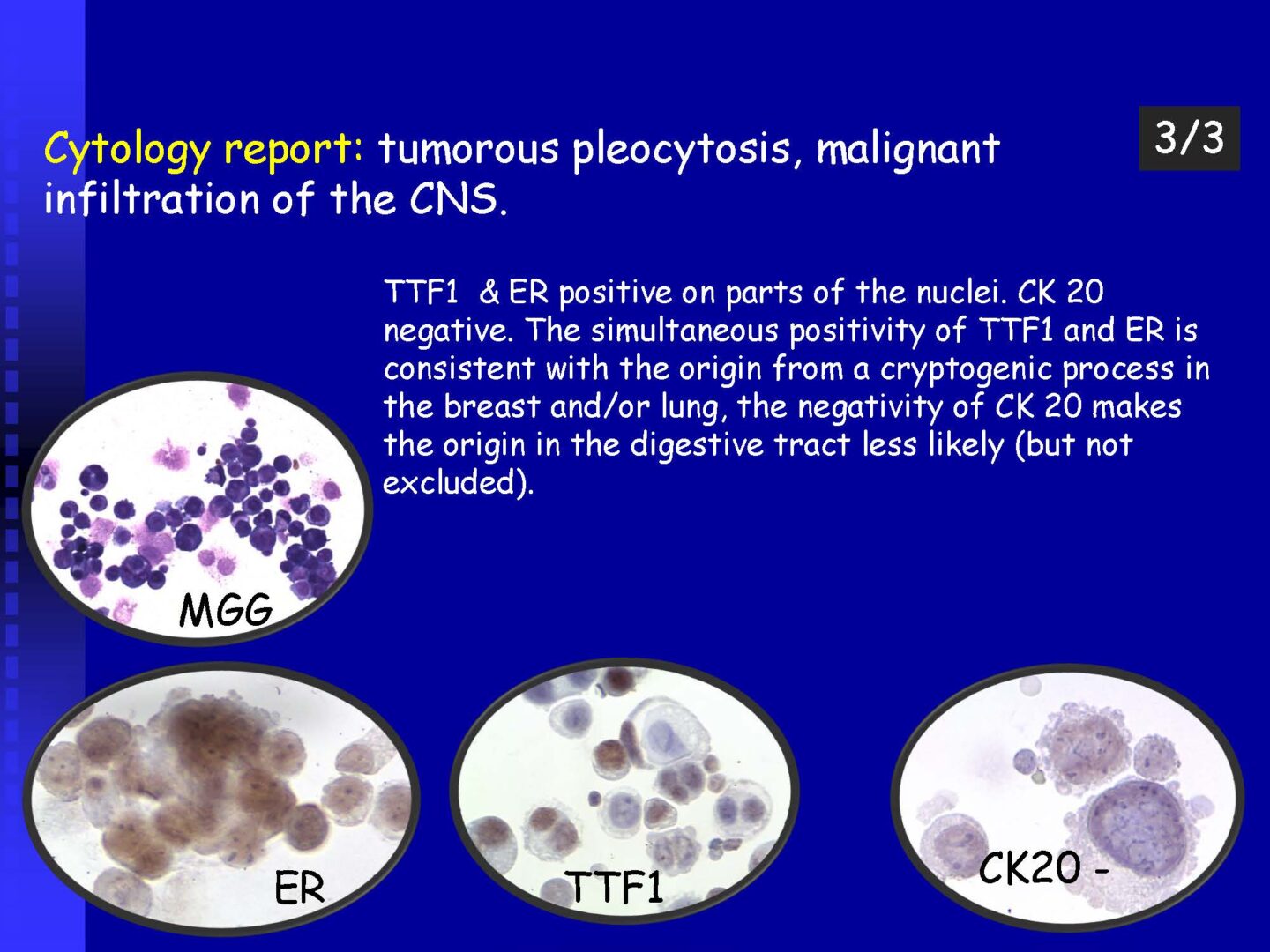
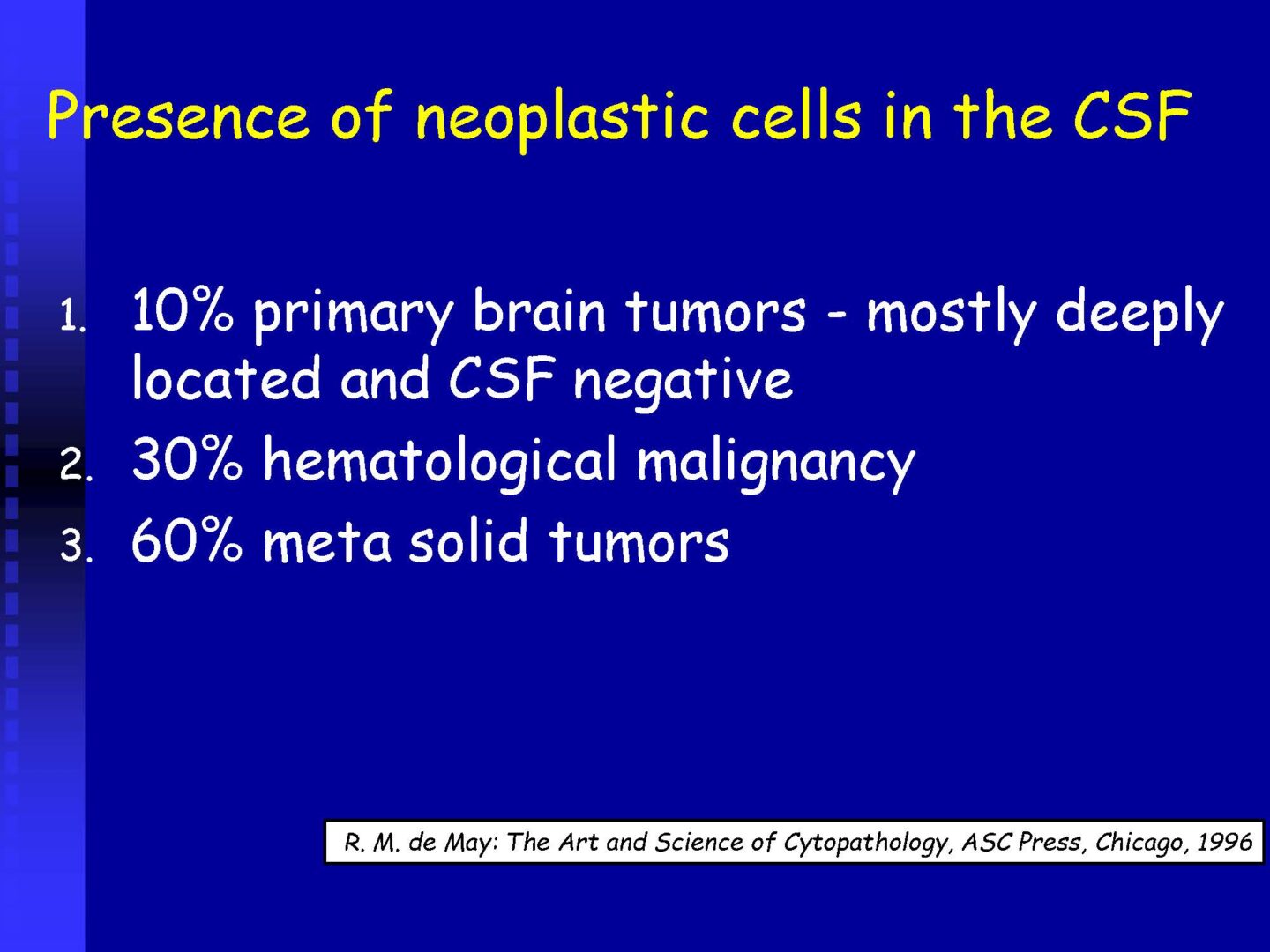
Neoplastic cells can be detected in the cerebrospinal fluid in case of tumor involvement of the CNS, whether metastatic or in primary CNS tumors, or in the context of the spread of hematological malignancies.The rate of detection of tumor cells in the cerebrospinal fluid depends on the location of the tumor in relation to the cerebrospinal fluid pathways, and/or degree of its cohesion, i.e. the ability to release individual tumor cells or their clusters into the cerebrospinal fluid compartment. Carcinomatosis of the meninges, or more precisely, malignant or carcinomatous meningeal infiltration, refers to a condition where malignant cells infiltrate the meninges without a detectable locus of metastasis in the CNS parenchyma and are released and detected in the cerebrospinal fluid.
Summary of classification & terminology for the cytopathological findings in the CSF see table 4.
Table 4. Basic classification & terminology of cytopathological findings in CSF
Oligocytosis – pathological condition with normal number of elements | ||
Type of oligocytosis | Characteristic | Dg. importance |
Lymphocytic | Activated lymphocytes, especially plasma cells | Serous inflammation, eg MS |
Monocytic | Activated cells of the monocyto-macrophage system | Terminal phase of neuroinfections. Cleaning reaction after bleeding or tissue damage, AIDP/CIDP, reaction to the tumor process |
Granulocytic | – with a predominance of neutrophil granulocytes | Initial phase of serous neuroinfections, reaction to acute tissue damage, bleeding |
Granulocytic | – with a predominance of eosinophilic granulocytes | mycotic, parasitic diseases, reaction to foreign material in the cerebrospinal tract (drain) |
Neoplastic | Tumor elements | Tumor affection of the CNS – further identification of tumor elements is necessary |
Pleocytosis – pathological condition with increased number of elements | ||
Type of pleocytosis | Characteristic | Dg. importance |
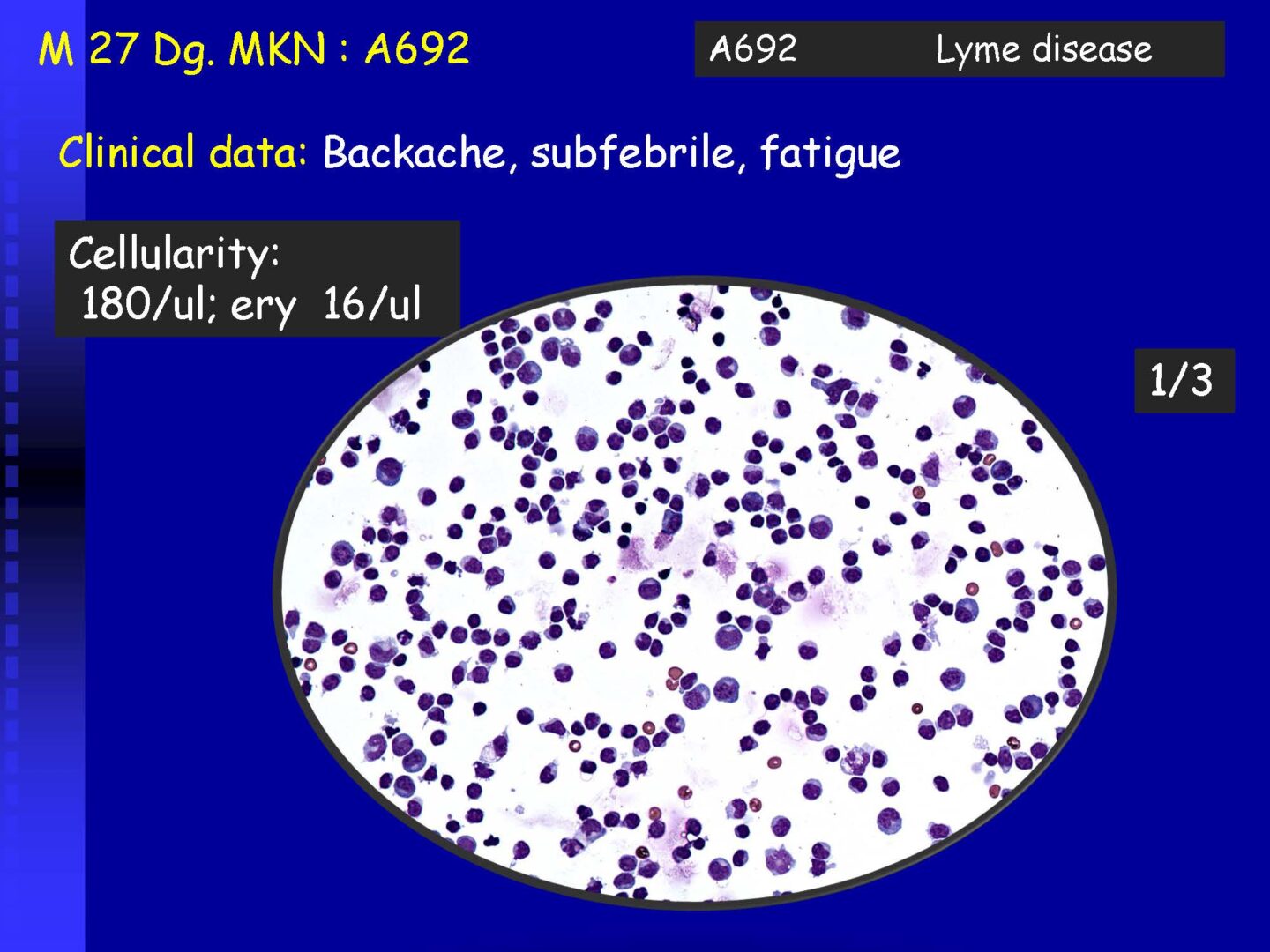
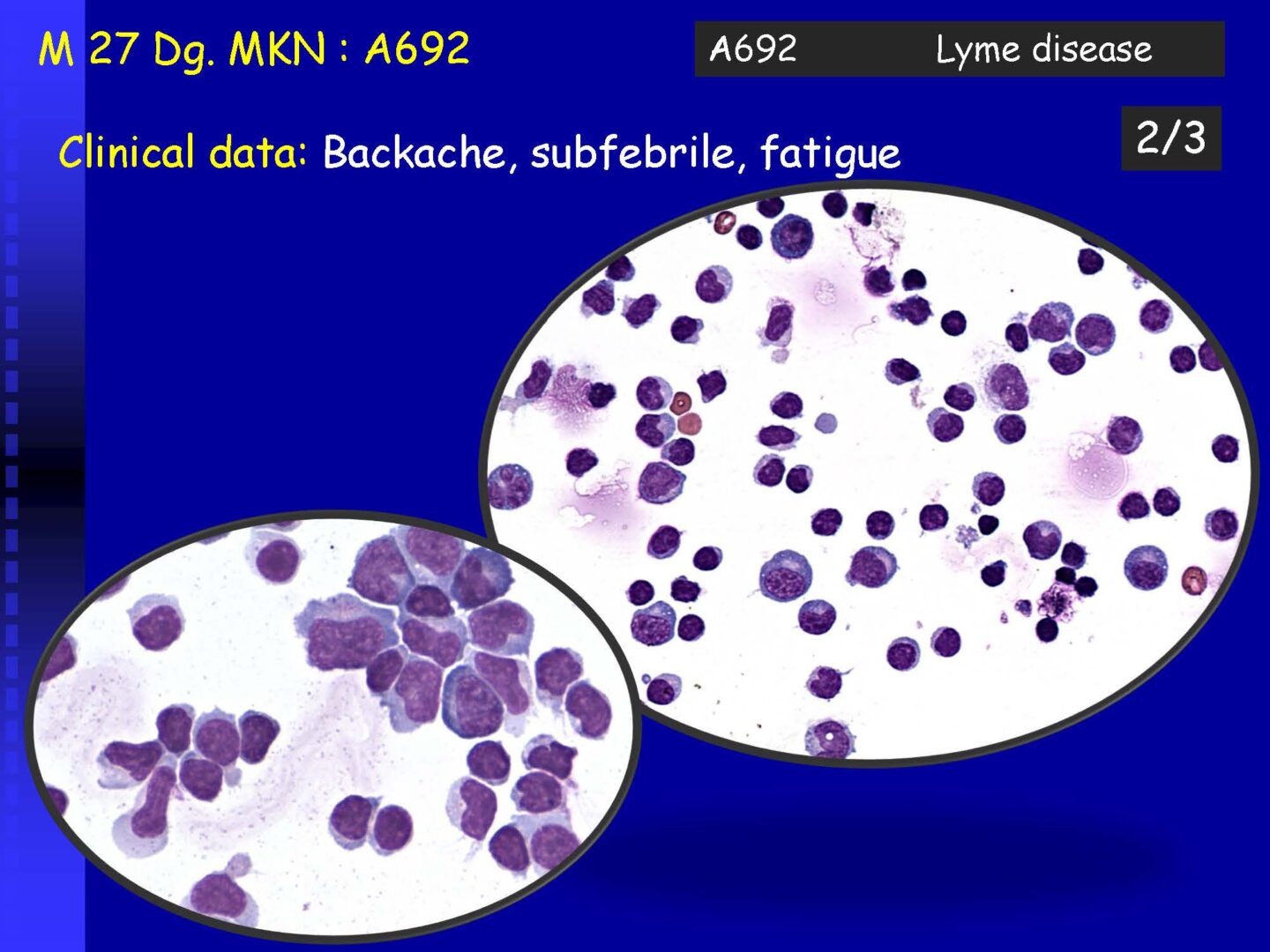
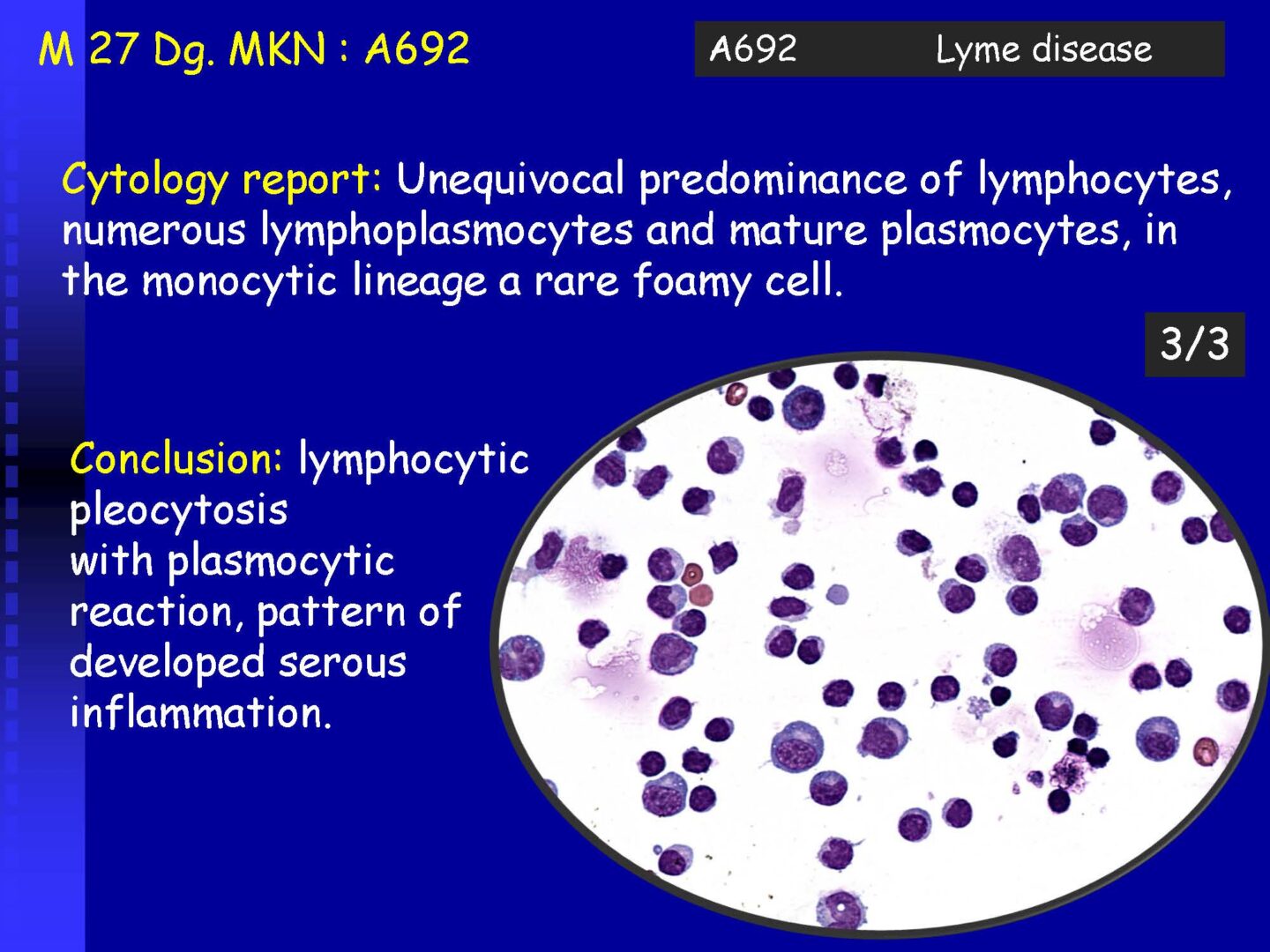
Lymphocytic (neuroboreliosis, neurolues, leptospirosis), but also autoimmune (e.g. MS). | Activated lymphocytes, especially mature plasma cells | Serous inflammations – viral neuroinfections, some bacterial-spirochete infections |
Monocytic | Activated cells of the monocyto-macrophagic system, macrophages | Cleaning reaction – e.g. after bleeding or tissue damage (ischemia, bleeding, neurosurgery, etc.), reaction to a tumor process, etc. |
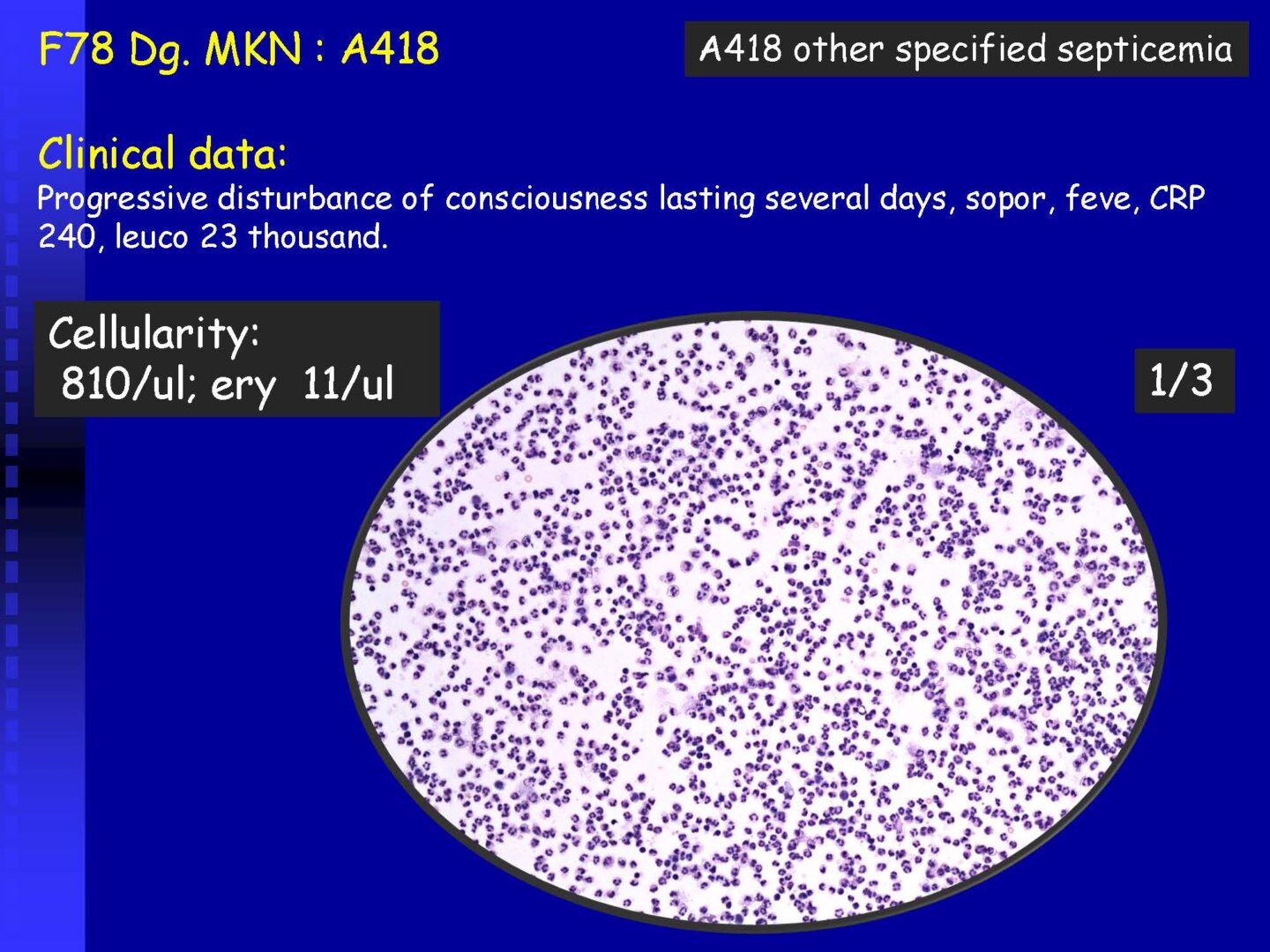
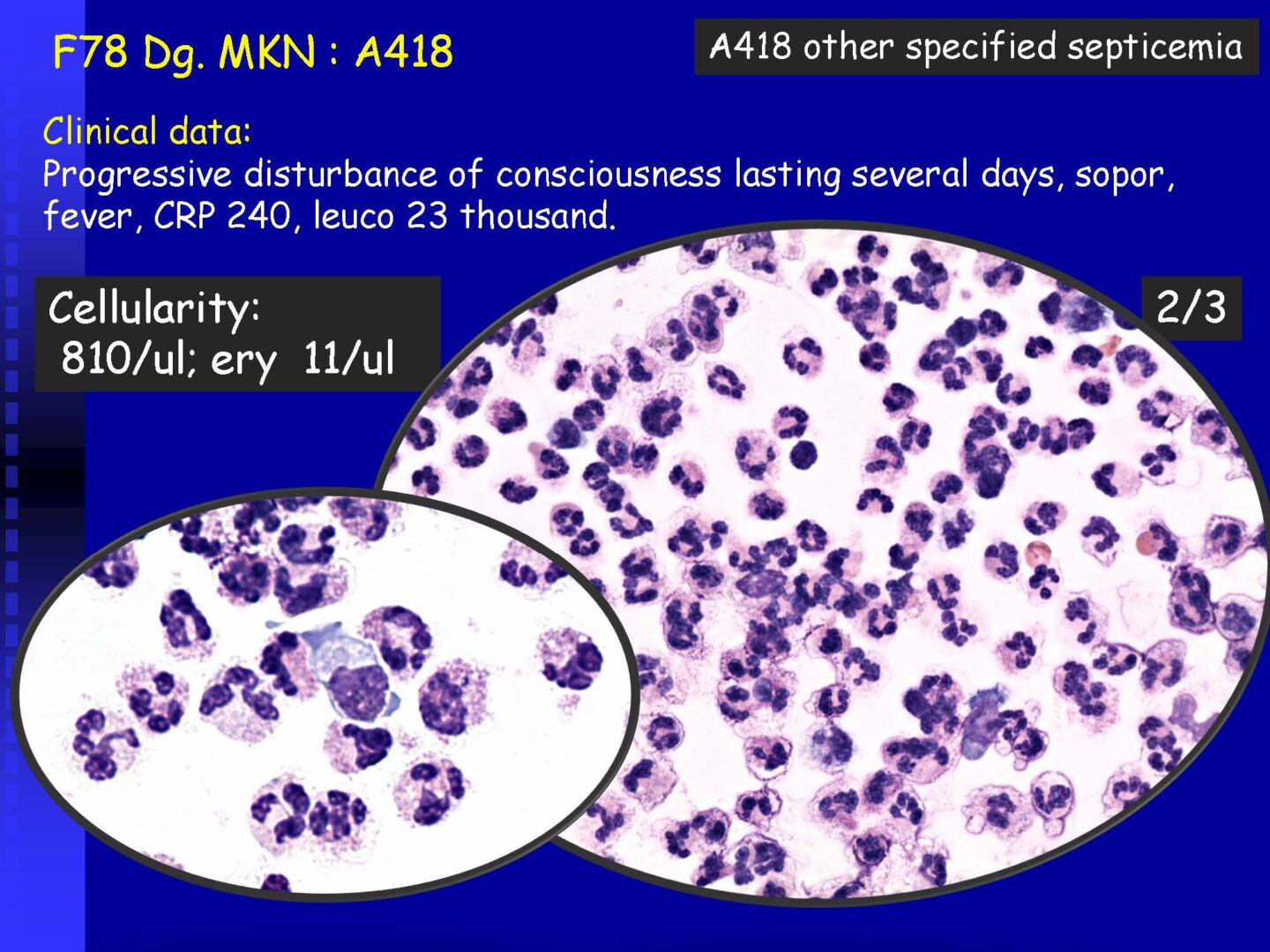
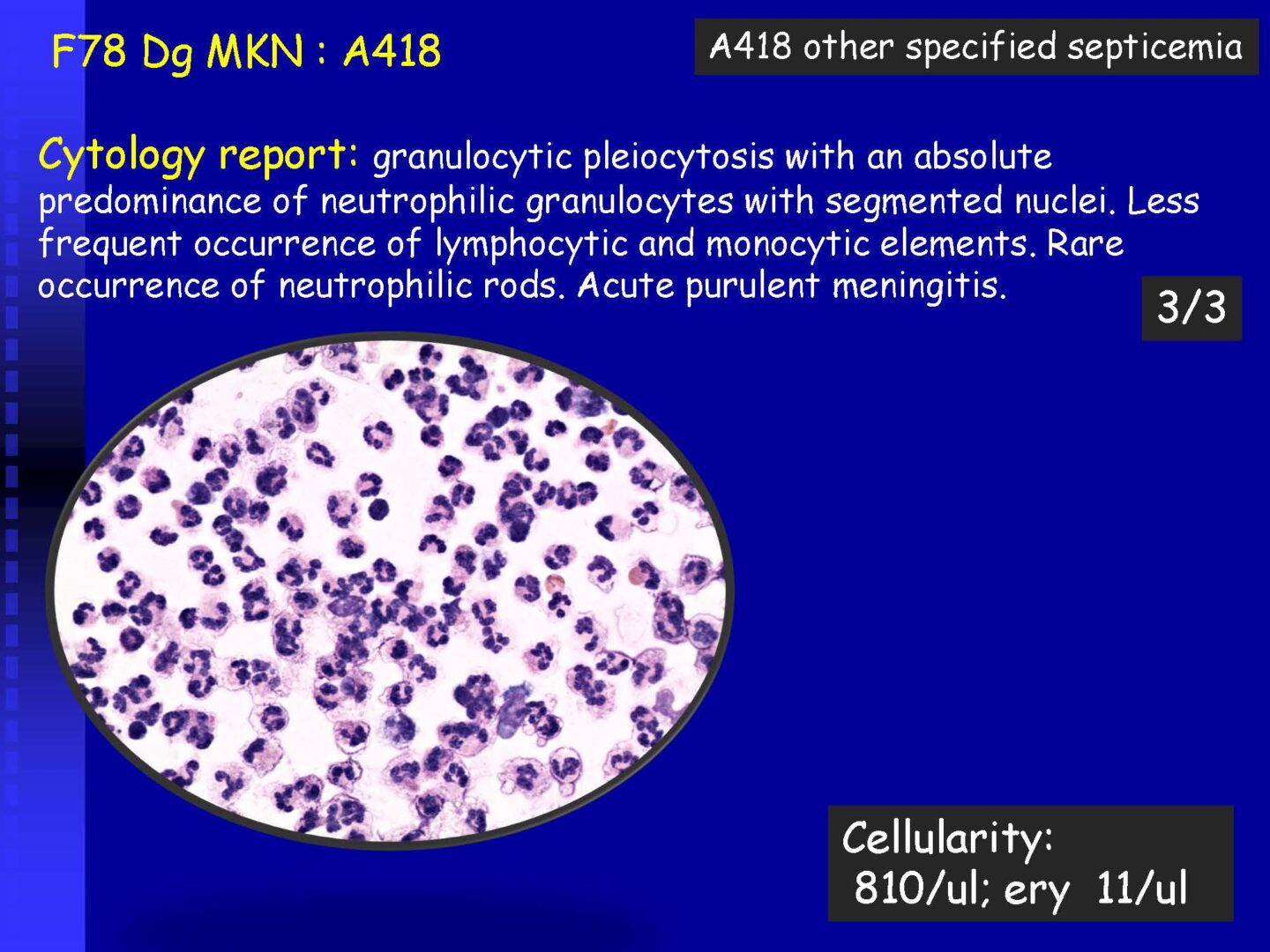
Granulocytic | – with a predominance of neutrophil granulocytes | Purulent meningitis – usually a bacterial neuroinfection (to assess the oxidative burst of PMN – see KEB parameter) |
Granulocytic | – with a predominance of eosinophilic granulocytes | Mycotic, parasitic diseases, reaction to foreign material in the CSF tracts (drain) |
Neoplastic | Tumor elements | – further identification of tumor elements is necessary |
For the detection of neoplastic cells – see details in CSF – analytical phase.
Quantitative cytology – cellularity assessment
Considering the physiological oligocellular nature of the CSF, cellularity assessment represents an important part of the diagnosis.
Fuchs – Rosenthal (FR) chamber (Fig. 4A,B):
The FR chamber has an area of 4×4 mm, a depth of 0.2 mm, so its volume is 3.2 µl (microliter).
Alternatively, a Bürker chamber (Fig. 5A,B) used to determine the cellularity of other liquid samples can be used.
Bürker’s chamber is significantly smaller overall – 3×3 mm in size and only 0.1 mm deep, so its total volume is 0.9 ul (microliter).
Cellularity assessment – method
Mix 100 µl of cerebrospinal fluid/sample and 10 µl of staining solution in a test tube, shake carefully.
Using a pipette, carefully apply the mixture to the Fuchs-Rosenthal counting chamber under the placed cover glass.
The appropriate time for counting is about 5 to 10 minutes after careful mixing (with a higher blood admixture, it is recommended to extend the time to complete hemolysis of erythrocytes).
Nuclear elements can also be counted from native material – staining is recommended mainly with a higher artificial blood admixture or with an increased amount of impurities.
Divide the result of counting the entire chamber by 3 and give the cellularity per 1 ul.
(Source: Laboratory manual, Topelex, s.r.o. –http://www.likvor.cz )
Automated methods based on flow cytometry are becoming increasingly widespread for cell counting in CSF – e.g. many automated hematology analyzers already offer a special mode for “CSF cell count”.
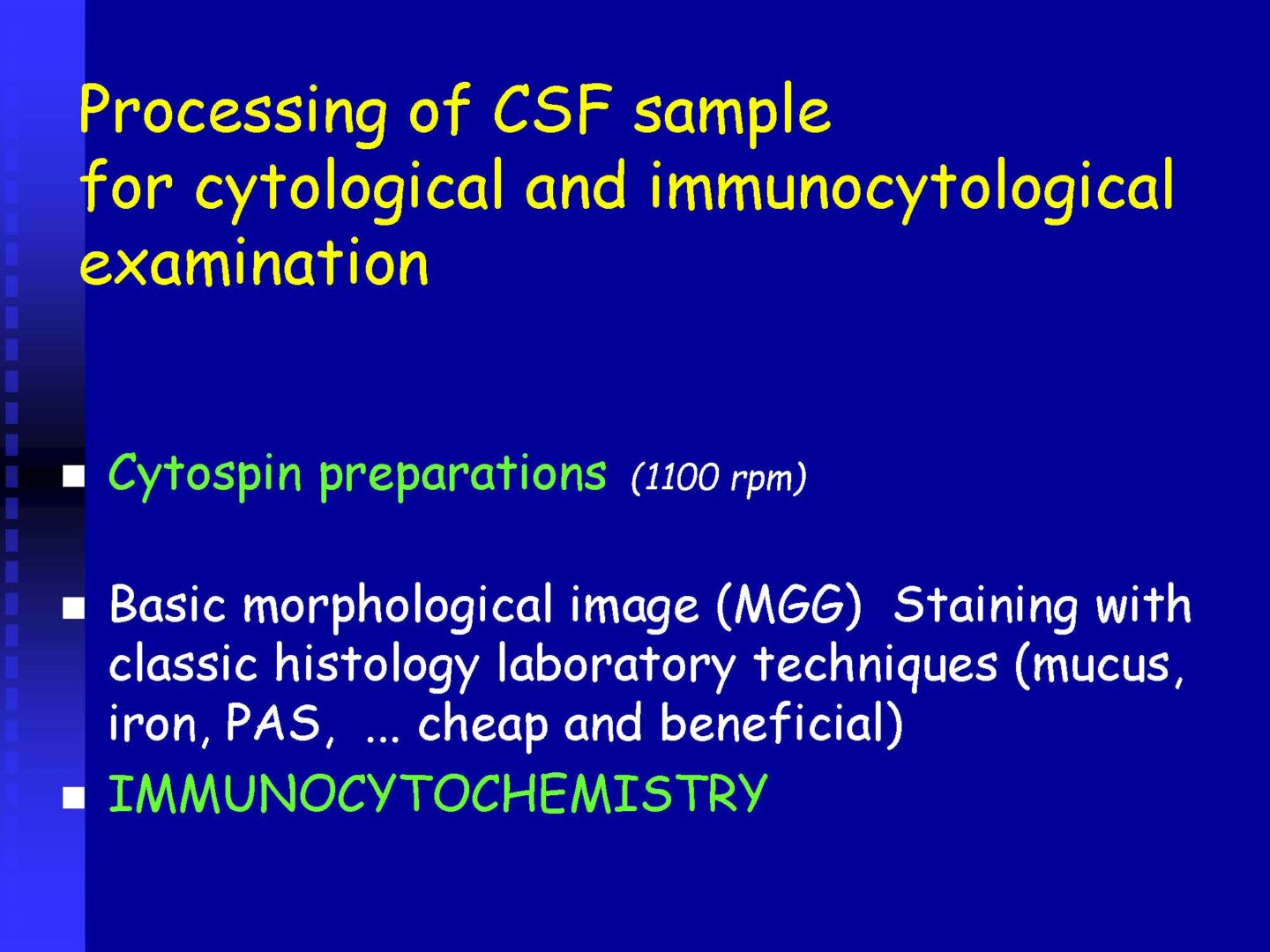
Qualitative cytology
Preparation of a permanent slide – cytocentrifugation
Typically 500 µl of material is processed
300 µl processed for ERYs above 3000, 250 µl for ERYs above 10,000.
Cytocentrifugation 5 min. / 11000 revolutions
Staining
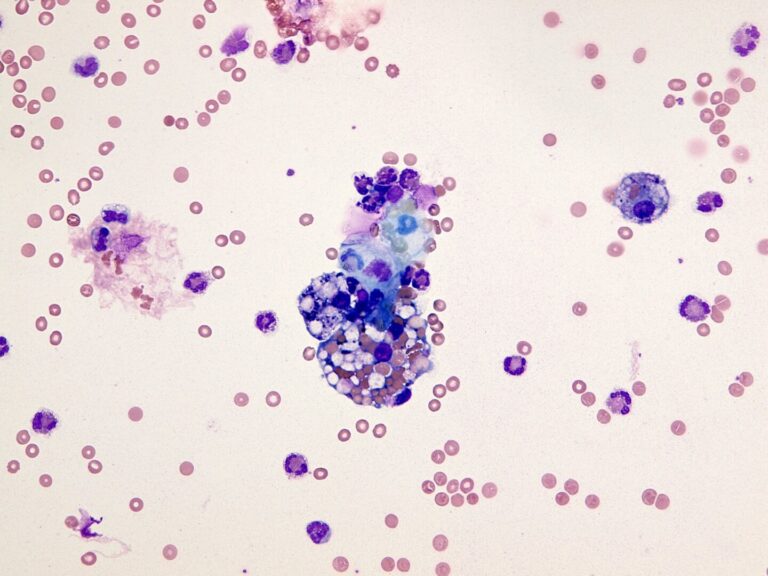
May-Grünwald-Giemsa – MGG ( Fig. 6), Oil Red O – ORO (Fig. 7A,B, Perls´reaction – Fe (Fig. 8) ….
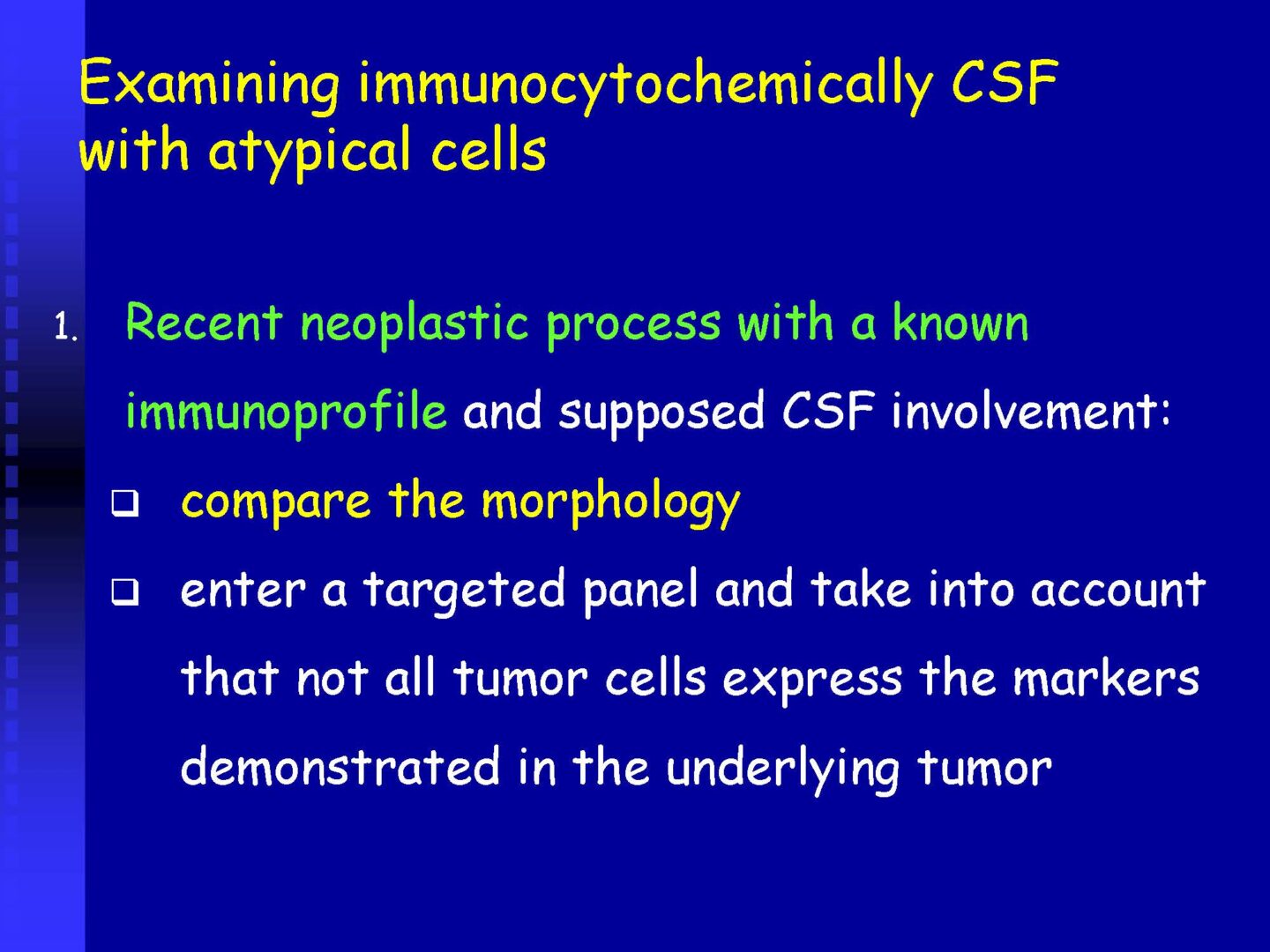
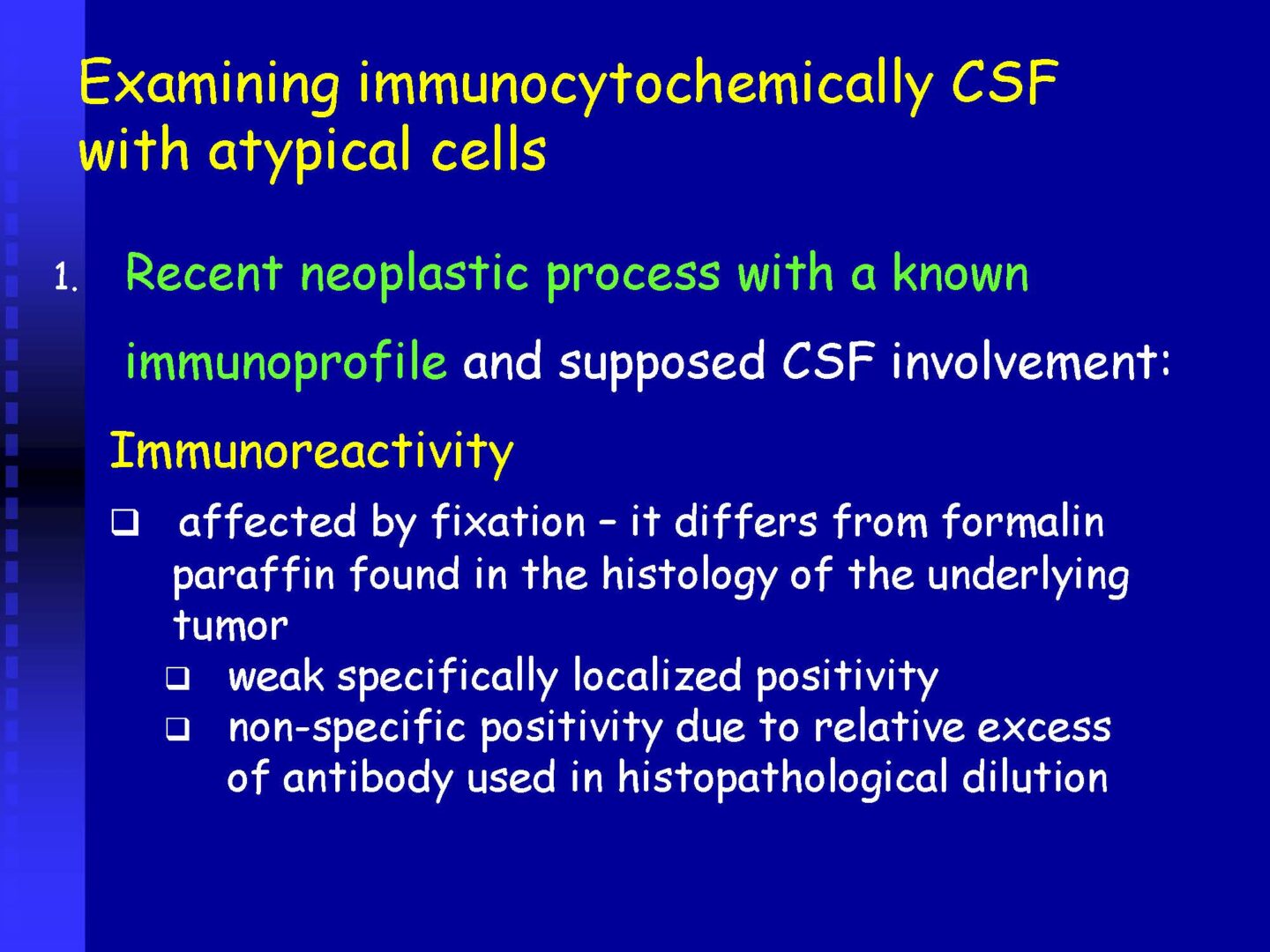
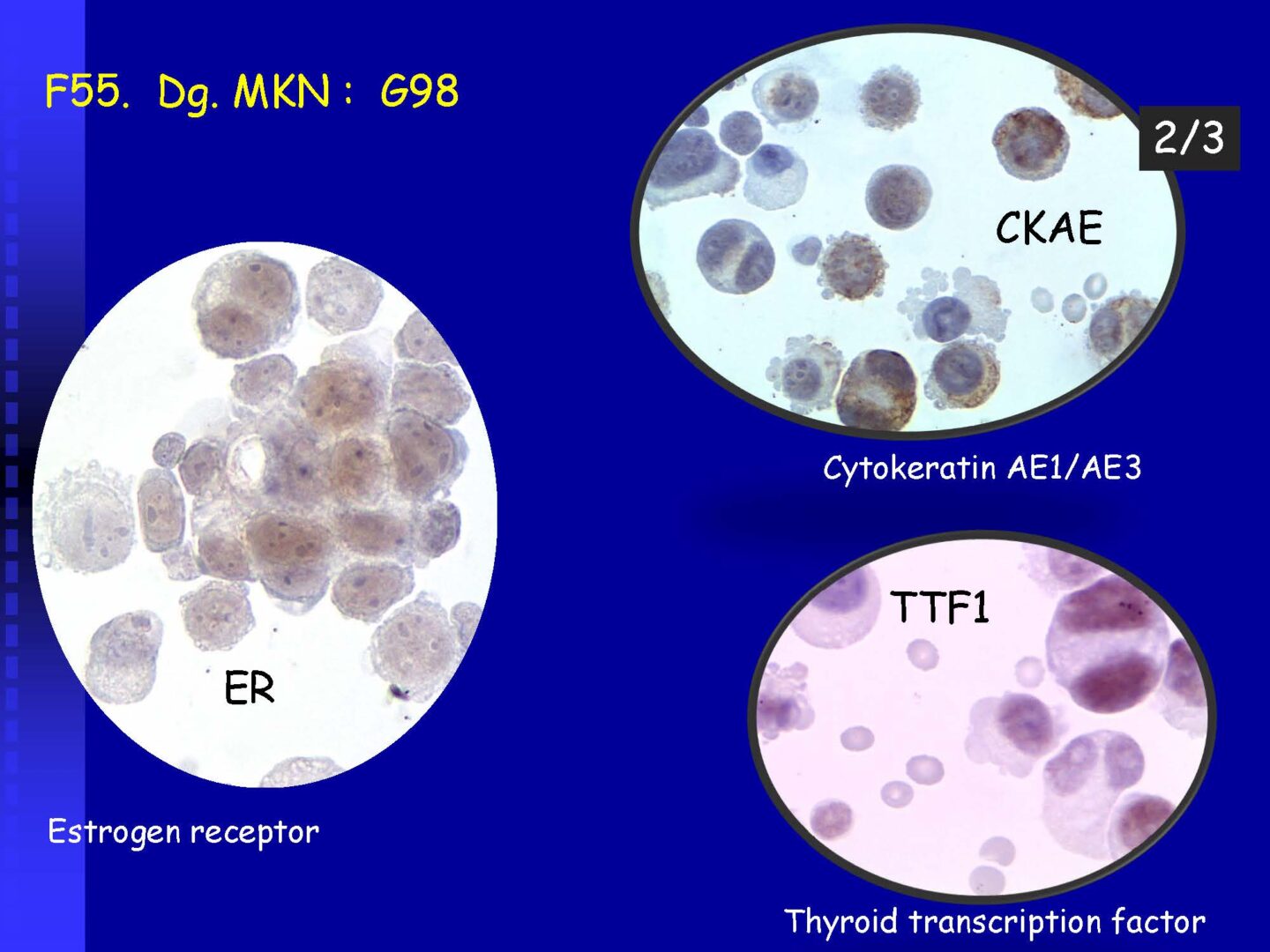
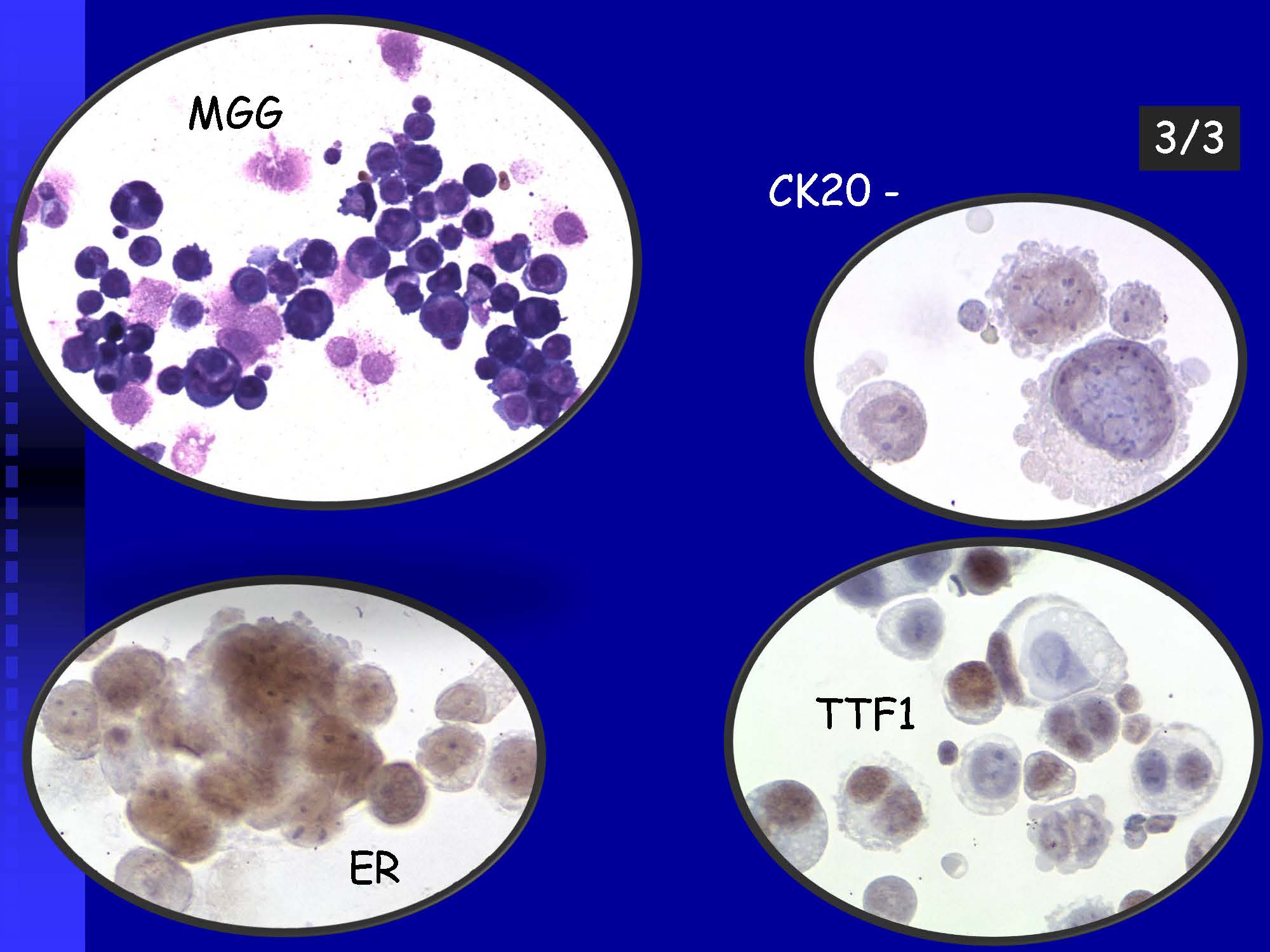
Detection of tumor elements in the cerebrospinal fluid, qualitative cytological examination, followed by immunocytochemical (ICC) identification of tumor cells using monoclonal antibodies, is irreplaceable. In case of infiltration of the CNS by hematological tumors, it is advisable to supplement the examination of the cerebrospinal fluid with flow cytometry (FCM), however, the overall low number of cells in the given volume of the sample may be a limiting factor here.
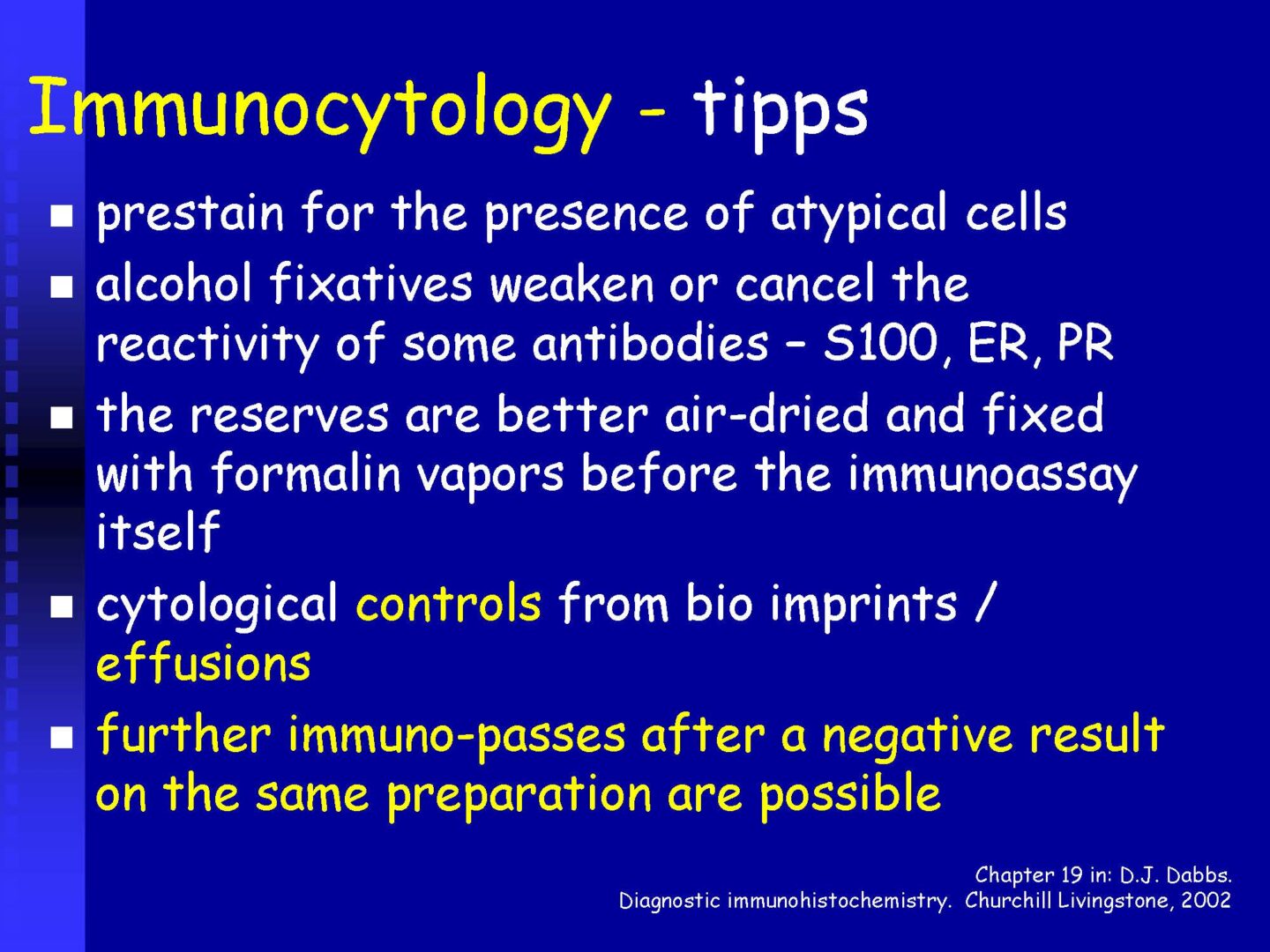
Immunocytochemistry
If malignancy is suspected, reserve preparations are prepared on silanized glasses and stained with MGG without mounting (depending on the availability of the supplied sample).
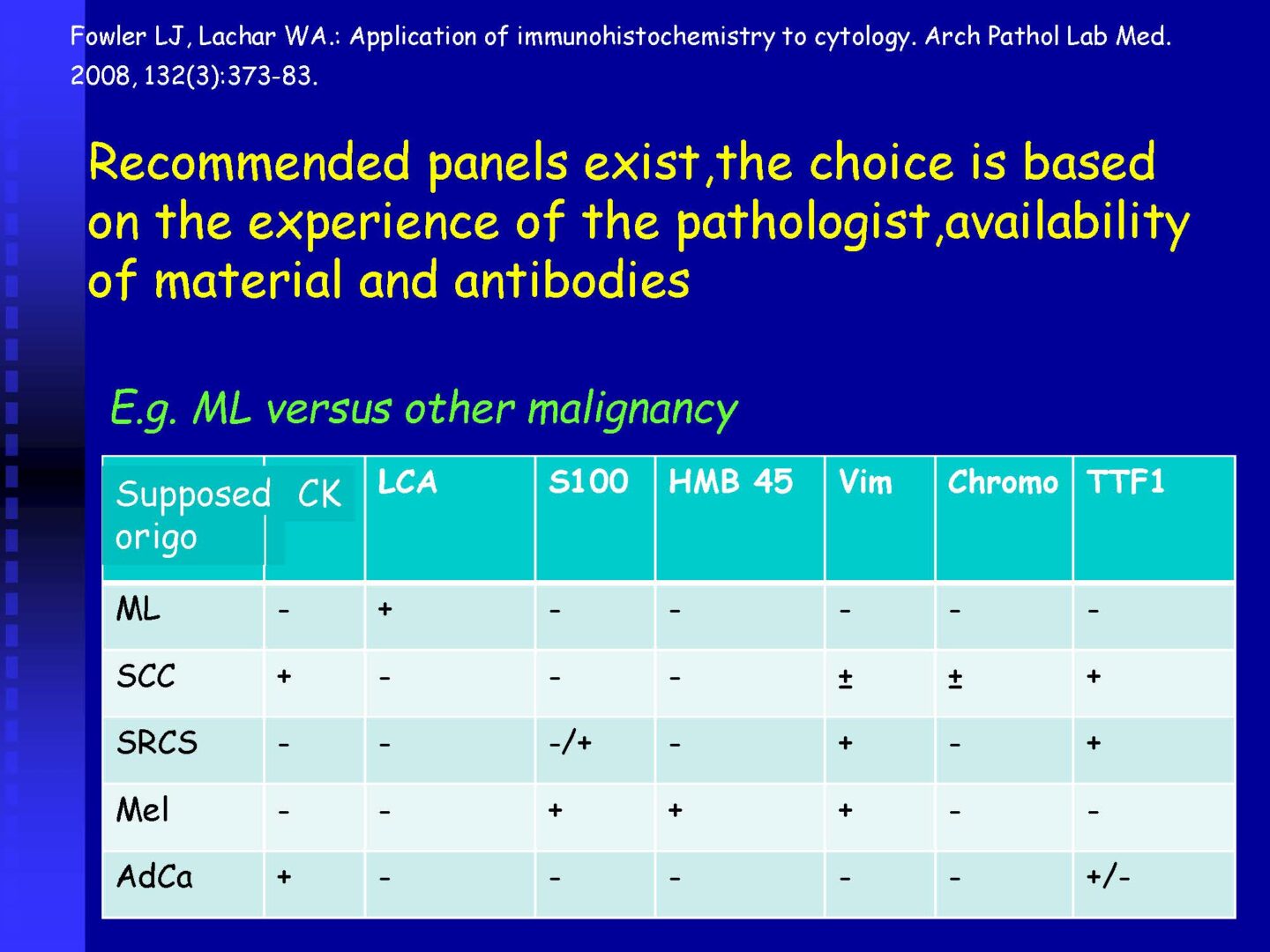
Procedures according to SOP (standard operation protocols) and antibody type using flexibility.
The laboratory regularly participates in the EQC [7-10]
[1] Sakka L, Coll G, Chazal J: Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 2011, 128:309-16.
[2] Felgenhauer, K.: Clinical Laboratory Diagnostics. TH-Books Verlag, Frankfurt am Main. 1998.
[3] Deisenhammer F, et al.: Guidelines on routine cerebrospinal fluid analysis. Report from EFNS taskforce. Eur J Neurol; 2006, 13(9): 913–922.
[4] Deisenhammer, F., Sellebjerg, F., Teunissen, C., Tumani, H. (eds) Cerebrospinal Fluid in Clinical Neurology. Springer, Cham. https://doi.org/10.1007/978-3-319-01225-4_6
[5] H. Tumani, H. F. Petereit, A. Gerritzen, C. C. Gross, A. Huss, S. Isenmann, S. Jesse, M. Khalil, P. Lewczuk, J. Lewerenz, F. Leypoldt, N. Melzer, S. G. Meuth, M. Otto, K. Ruprecht, E. Sindern, A. Spreer, M. Stangel, H. Strik, M. Uhr, J. Vogelgsang, K.-P. Wandinger, T. Weber, M. Wick, B. Wildemann, J. Wiltfang, D. Woitalla, I. Zerr, T. Zimmermann, S1 guidelines “lumbar puncture and cerebrospinal fluid analysis”, Neurological Research and Practice, 10.1186/s42466-020-0051-z, 2, 1, (2020).
[6] Perske C, Nagel I, Nagel H, Strik H. CSF cytology–the ongoing dilemma to distinguish neoplastic and inflammatory lymphocytes. Diagn Cytopathol. 2011 Aug;39(8):621-6. doi: 10.1002/dc.21510. Epub 2010 Nov 22. PMID: 21761583.
[7] Dušková J, Sobek O.: Assisting the neurologist in diagnosis of CNS malignancies. Brain Behav.; 2017,e00805. https://doi.org/10.1002/brb3.805
[8] Schmitt F, Cochand-Priollet B, Toetsch M, Davidson B, Bondi A, Vielh P. Immunocytochemistry in Europe: results of the European Federation of Cytology Societies (EFCS) inquiry. Cytopathology. 2011 Aug;22(4):238-42. doi: 10.1111/j.1365-2303.2011.00885.
[9] Srebotnik Kirbiš I, Rodrigues Roque R, Bongiovanni M, Strojan Fležar M, Cochand-Priollet B. Immunocytochemistry practices in European cytopathology laboratories-Review of European Federation of Cytology Societies (EFCS) online survey results with best practice recommendations. Cancer Cytopathol. 2020 Oct;128(10):757-766. doi: 10.1002/cncy.22311. Epub 2020 Jun 29
[10] Kirbis IS, Maxwell P, Flezar MS, Miller K, Ibrahim M. External quality control for immunocytochemistry on cytology samples: a review of UK NEQAS ICC (cytology module) results. Cytopathology. 2011;22(4):230-237.